Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2020-11-21 , DOI: 10.1016/j.apcatb.2020.119712 Andoni Choya , Beatriz de Rivas , Juan Ramón González-Velasco , Jose Ignacio Gutiérrez-Ortiz , Rubén López-Fonseca
|
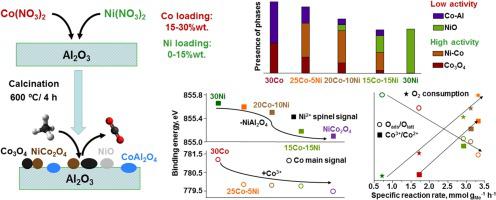
This work deals with the extensive study of supported bimetallic Co-Ni catalysts for the complete oxidation of methane. The simultaneous incorporation of nickel and cobalt was found to enhance the redox properties by favouring the formation of nickel cobaltite-like species and partially inhibiting the interaction between Co3O4 and alumina. In turn, this resulted in an increase of the amount of Co3+ cations in the catalysts, which led to a more notable presence of active lattice oxygen species in the samples. When the nickel loading was increased, the generation of less active NiO was observed. The improvement of the redox properties resulted in a promotion of the specific reaction rate and a shift of around 50 °C in the T50 value over the bimetallic catalysts. The most active catalyst (25Co-5Ni) was found to be relatively stable during prolonged operation times, but suffered an appreciable and irreversible deactivation in the presence of water vapour.
中文翻译:

天然气车辆中甲烷氧化用双金属Co-Ni负载催化剂的优化。
这项工作涉及对甲烷完全氧化的负载型双金属Co-Ni催化剂的广泛研究。发现镍和钴的同时掺入通过促进镍状钴样物质的形成并部分抑制Co 3 O 4与氧化铝之间的相互作用而增强了氧化还原性质。反过来,这导致催化剂中Co 3+阳离子的数量增加,这导致样品中活性晶格氧物种的存在更加明显。当镍负载增加时,观察到活性较低的NiO的产生。氧化还原性能的改善导致比反应速率的提高和T 50的转变约50°C价值超过双金属催化剂。发现活性最强的催化剂(25Co-5Ni)在延长的操作时间中相对稳定,但是在水蒸气存在下会发生明显且不可逆的失活。



























 京公网安备 11010802027423号
京公网安备 11010802027423号