当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
DNA Nanodevices for Base Excision Repair Regulates ATP In Situ Imaging and Tumor Therapy
ACS Applied Bio Materials ( IF 4.7 ) Pub Date : 2020-11-20 , DOI: 10.1021/acsabm.0c00884 Yao Jiang 1, 2 , Xiangjuan Kong 3 , Yanxialei Jiang 2 , Wenjing Zhao 2 , Huimin Zhou 2 , Shusheng Zhang 2
ACS Applied Bio Materials ( IF 4.7 ) Pub Date : 2020-11-20 , DOI: 10.1021/acsabm.0c00884 Yao Jiang 1, 2 , Xiangjuan Kong 3 , Yanxialei Jiang 2 , Wenjing Zhao 2 , Huimin Zhou 2 , Shusheng Zhang 2
Affiliation
|
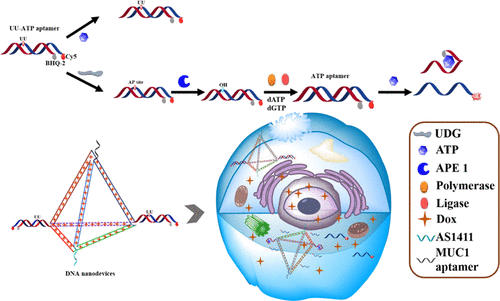
The design of DNA nanodevices has attracted broad attention in detecting specific targets and targeted drug delivery capacities of tumor cells. Here, we report the facile fluorometric method of dual-targeting DNA nanodevices for base excision repair (BER) regulates adenosine triphosphate (ATP) in situ imaging and tumor therapy that can counteract the mutagenic effects of uracil (U) on ATP aptamer based on the binding of U-containing damaged ATP aptamer. We prove that the DNA nanodevices not only effectively deliver the aptamer probe and tumor therapy but also able to analyze the overexpression of APE1 and uracil-DNA glycosylases (UDG) in the BER pathway via ATP in situ imaging in tumor cells. Therefore, the DNA nanodevices of the BER pathway provide the potential for tumor theranostics.
中文翻译:

用于碱基切除修复的 DNA 纳米装置调节 ATP 原位成像和肿瘤治疗
DNA纳米器件的设计在检测肿瘤细胞的特定靶点和靶向药物递送能力方面引起了广泛关注。在这里,我们报告了用于碱基切除修复 (BER) 的双靶向 DNA 纳米装置的简便荧光法调节三磷酸腺苷 (ATP) 原位成像和肿瘤治疗,可以抵消尿嘧啶 (U) 对 ATP 适体的诱变作用。含 U 受损 ATP 适体的结合。我们证明 DNA 纳米器件不仅可以有效地提供适体探针和肿瘤治疗,而且能够通过肿瘤细胞中的 ATP 原位成像分析 APE1 和尿嘧啶-DNA 糖基化酶 (UDG) 在 BER 途径中的过表达。因此,BER 通路的 DNA 纳米器件为肿瘤治疗学提供了潜力。
更新日期:2020-12-21
中文翻译:

用于碱基切除修复的 DNA 纳米装置调节 ATP 原位成像和肿瘤治疗
DNA纳米器件的设计在检测肿瘤细胞的特定靶点和靶向药物递送能力方面引起了广泛关注。在这里,我们报告了用于碱基切除修复 (BER) 的双靶向 DNA 纳米装置的简便荧光法调节三磷酸腺苷 (ATP) 原位成像和肿瘤治疗,可以抵消尿嘧啶 (U) 对 ATP 适体的诱变作用。含 U 受损 ATP 适体的结合。我们证明 DNA 纳米器件不仅可以有效地提供适体探针和肿瘤治疗,而且能够通过肿瘤细胞中的 ATP 原位成像分析 APE1 和尿嘧啶-DNA 糖基化酶 (UDG) 在 BER 途径中的过表达。因此,BER 通路的 DNA 纳米器件为肿瘤治疗学提供了潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号