Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Efficient Substrate-Induced Method for the Synthesis of CF3-Substituted Cyclopropanes by Metal-Free Reaction of Trifluoromethyl Styrylisoxazoles with Nitromethane
Synthesis ( IF 2.6 ) Pub Date : 2020-11-19 , DOI: 10.1055/s-0040-1705976 Feng Li 1 , Hai Ma 2 , Qing-he Zhao 2 , Guang-hao Yu 2 , Ye-chen Meng 3 , Feng Sui 2 , Mi-yi Yang 2 , Li Liu 2 , Hui-jie Li 2
中文翻译:

三氟甲基苯乙烯基恶唑与硝基甲烷的无金属反应合成CF3取代的环丙烷的高效底物诱导方法
更新日期:2020-11-21
Synthesis ( IF 2.6 ) Pub Date : 2020-11-19 , DOI: 10.1055/s-0040-1705976 Feng Li 1 , Hai Ma 2 , Qing-he Zhao 2 , Guang-hao Yu 2 , Ye-chen Meng 3 , Feng Sui 2 , Mi-yi Yang 2 , Li Liu 2 , Hui-jie Li 2
Affiliation
|
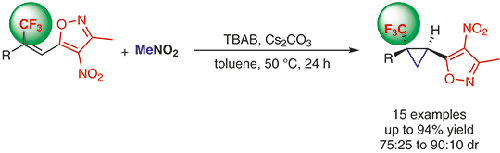
A series of (trifluoromethyl)cyclopropanes (TFCPs) tolerating a broad range of functional groups, known as tert-butyl bioisosteres, have been obtained from the cyclization reaction between nitromethane and 5-[β-(trifluoromethyl)styryl]isoxazoles in 70–94% yields and 75:25 to 90:10 dr. This method offers practical access to this cyclopropyl moiety of pharmacological interest, employing an inorganic base under phase-transfer conditions.
中文翻译:

三氟甲基苯乙烯基恶唑与硝基甲烷的无金属反应合成CF3取代的环丙烷的高效底物诱导方法
从硝基甲烷和5- [β-(三氟甲基)苯乙烯基]异恶唑在70-94之间的环化反应中获得了一系列耐受广泛官能团的(三氟甲基)环丙烷(TFCP),称为叔丁基生物等排体。 %产率和75:25至90:10 dr。通过在相转移条件下使用无机碱,该方法提供了实用的药理学意义上的环丙基部分的实用途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号