当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic Expression for Optimal Catalyst Electronic Configuration: The Case of Ammonia Decomposition
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-11-18 , DOI: 10.1021/acs.jpcc.0c08432 Nigora Turaeva 1 , Rebecca Fushimi 2 , Gregory Yablonsky 3
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-11-18 , DOI: 10.1021/acs.jpcc.0c08432 Nigora Turaeva 1 , Rebecca Fushimi 2 , Gregory Yablonsky 3
Affiliation
|
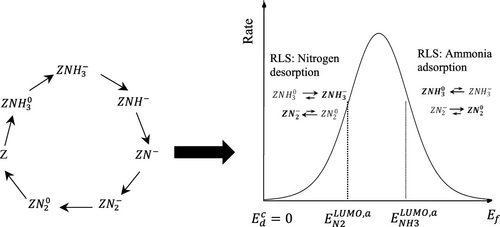
A new steady-state kinetic model of ammonia decomposition is presented and analyzed regarding the electronic properties of metal catalysts. The model is based on the classical Temkin–Ertl mechanism and modified in accordance with Wolkenstein’s electronic theory by implementing participation of free electrons of the catalyst to change the chemical nature of adsorbed species. Wolkenstein’s original theory only applied to semiconductors, but by including the d-band model for splitting of adsorbate molecular orbitals into bonding/antibonding states, the electronic theory can be extended to metals. The relative population of charged versus neutral adsorbates is a function of the Fermi level of the catalyst and the d-band splitting of the adsorbate. Moreover, charged and neutral adsorbates will present different reactivities and the overall reaction rate can be described as a function of the Fermi level. For both simplified and full reaction mechanisms, including electronic steps, we present a steady-state rate equation where the dependence on the Fermi level of the metal creates a volcano-shaped dependence. The detailed kinetic model is compared to recent experimental measurements of ammonia decomposition on iron, cobalt, and CoFe bimetallic catalysts. This result agrees with the classical qualitative Sabatier statement and the quantitative theoretical results of the optimal binding energy presented by Nørskov and co-workers. In contrast to the classical volcano curves, considering a single reactant, the volcano-shaped reaction rate of the presented model is obtained via the interplay of both reactant adsorption and product desorption, which is achieved by considering the role of electronic subsystems. Previously, the role of the electronic subsystem has been disregarded because it consists of fast reactions. In our analysis, it is shown that despite fast electronic kinetic parameters, the role of the equilibrium constants could not be disregarded. These terms give rise to the volcano-type dependence.
中文翻译:

最佳催化剂电子构型的动力学表达:氨分解的情况
提出了一种新的氨分解稳态动力学模型,并对金属催化剂的电子性质进行了分析。该模型基于经典的Temkin-Ertl机理,并根据Wolkenstein的电子理论进行了修改,通过实现催化剂的自由电子的参与来改变吸附物质的化学性质。Wolkenstein的原始理论仅适用于半导体,但是通过包含将吸附物分子轨道分裂为键合/反键态的d带模型,电子理论可以扩展到金属。收费的相对人数与中性吸附物是催化剂费米能级和吸附物d带分裂的函数。而且,带电和中性的吸附物将具有不同的反应性,总反应速率可以描述为费米能级的函数。对于简化的反应机理和完整的反应机理(包括电子步骤),我们提出了一个稳态速率方程,其中对金属费米能级的依赖性导致了火山状的依赖性。将详细的动力学模型与最近在铁,钴和CoFe双金属催化剂上进行氨分解的实验测量值进行了比较。该结果与经典的定性Sabatier陈述以及Nørskov及其同事提出的最佳结合能的定量理论结果相吻合。与经典的火山曲线相比,通过反应物吸附和产物解吸的相互作用,这是通过考虑电子子系统的作用来实现的。以前,电子子系统的作用已被忽略,因为它由快速反应组成。在我们的分析中显示,尽管电子动力学参数快速,但平衡常数的作用不可忽视。这些术语引起了火山类型的依赖性。
更新日期:2020-12-03
中文翻译:

最佳催化剂电子构型的动力学表达:氨分解的情况
提出了一种新的氨分解稳态动力学模型,并对金属催化剂的电子性质进行了分析。该模型基于经典的Temkin-Ertl机理,并根据Wolkenstein的电子理论进行了修改,通过实现催化剂的自由电子的参与来改变吸附物质的化学性质。Wolkenstein的原始理论仅适用于半导体,但是通过包含将吸附物分子轨道分裂为键合/反键态的d带模型,电子理论可以扩展到金属。收费的相对人数与中性吸附物是催化剂费米能级和吸附物d带分裂的函数。而且,带电和中性的吸附物将具有不同的反应性,总反应速率可以描述为费米能级的函数。对于简化的反应机理和完整的反应机理(包括电子步骤),我们提出了一个稳态速率方程,其中对金属费米能级的依赖性导致了火山状的依赖性。将详细的动力学模型与最近在铁,钴和CoFe双金属催化剂上进行氨分解的实验测量值进行了比较。该结果与经典的定性Sabatier陈述以及Nørskov及其同事提出的最佳结合能的定量理论结果相吻合。与经典的火山曲线相比,通过反应物吸附和产物解吸的相互作用,这是通过考虑电子子系统的作用来实现的。以前,电子子系统的作用已被忽略,因为它由快速反应组成。在我们的分析中显示,尽管电子动力学参数快速,但平衡常数的作用不可忽视。这些术语引起了火山类型的依赖性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号