当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Densities and Apparent Molar Volumes of Aqueous Solutions of Zinc Sulfate at Temperatures from 293 to 373 K and 0.1 MPa Pressure
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-11-19 , DOI: 10.1021/acs.jced.0c00766 Lubomir Hnedkovsky 1 , Lea Rasanen 2 , Pertti Koukkari 2 , Glenn Hefter 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-11-19 , DOI: 10.1021/acs.jced.0c00766 Lubomir Hnedkovsky 1 , Lea Rasanen 2 , Pertti Koukkari 2 , Glenn Hefter 1
Affiliation
|
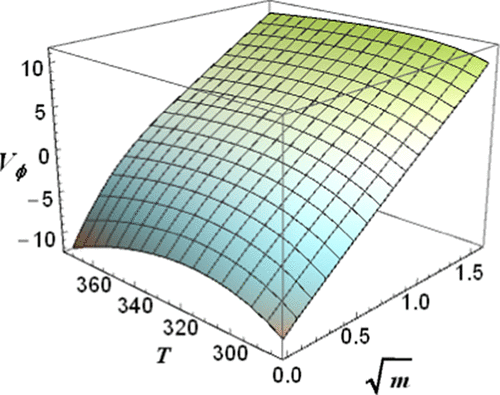
Densities of up to 16 aqueous solutions of zinc sulfate have been measured at 5 K intervals over the temperature range of 293.15 ≤ T/K ≤ 353.15 at concentrations 0.004 ≤ m/mol·kg–1 ≤ 2.5 and 0.1 MPa pressure using a commercial glass vibrating tube densimeter (vtd). Particular attention was paid to establishing the concentrations and pH values of the solutions. Densities of the same solutions were also measured at 343.15 and 373.15 K at 0.3 MPa pressure in a purpose-built high-temperature Pt/Rh-vtd. The two sets of densities at 343.15 K were in excellent agreement, with an average difference of 0.01%. Apparent molar volumes, Vϕ, calculated from the densities were fitted with an extended Redlich–Rosenfeld–Meyer equation. However, the standard (infinite dilution) molar volumes, Vo(ZnSO4, aq), derived via this equation differed significantly (by up to 2 cm3·mol–1) from the values obtained by ionic additivity using literature data. This difference is probably mostly due to ion-pairing effects.
中文翻译:

在293至373 K和0.1 MPa压力下硫酸锌水溶液的密度和表观摩尔体积。
向上的密度,以硫酸锌已被以5K间隔的温度范围内的293.15测量≤16水溶液Ť / K≤353.15在浓度0.004≤米/摩尔公斤· -1 ≤2.5和0.1兆帕压力使用市售的玻璃振动管密度计(vtd)。特别注意确定溶液的浓度和pH值。在特制的高温Pt / Rh-vtd中,还以0.3 MPa的压力在343.15和373.15 K下测量了相同溶液的密度。两组密度在343.15 K处非常吻合,平均差异为0.01%。表观摩尔体积,V ϕ根据密度计算的,与扩展的Redlich-Rosenfeld-Meyer方程拟合。但是,通过此方程式得出的标准(无限稀释)摩尔体积V o(ZnSO 4,aq)与使用文献数据通过离子加成法获得的值有显着差异(最大2 cm 3 ·mol –1)。这种差异可能主要归因于离子对效应。
更新日期:2021-01-14
中文翻译:

在293至373 K和0.1 MPa压力下硫酸锌水溶液的密度和表观摩尔体积。
向上的密度,以硫酸锌已被以5K间隔的温度范围内的293.15测量≤16水溶液Ť / K≤353.15在浓度0.004≤米/摩尔公斤· -1 ≤2.5和0.1兆帕压力使用市售的玻璃振动管密度计(vtd)。特别注意确定溶液的浓度和pH值。在特制的高温Pt / Rh-vtd中,还以0.3 MPa的压力在343.15和373.15 K下测量了相同溶液的密度。两组密度在343.15 K处非常吻合,平均差异为0.01%。表观摩尔体积,V ϕ根据密度计算的,与扩展的Redlich-Rosenfeld-Meyer方程拟合。但是,通过此方程式得出的标准(无限稀释)摩尔体积V o(ZnSO 4,aq)与使用文献数据通过离子加成法获得的值有显着差异(最大2 cm 3 ·mol –1)。这种差异可能主要归因于离子对效应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号