当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Total Synthesis of Pentacyclic Proaporphine Alkaloid (−)‐Misramine
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-11-18 , DOI: 10.1002/adsc.202001139 Liu‐Yang Pu 1 , Fan Yang 1 , Ji‐Qiang Chen 1 , Ying Xiong 1 , Jian‐Hua Xie 1 , Qi‐Lin Zhou 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-11-18 , DOI: 10.1002/adsc.202001139 Liu‐Yang Pu 1 , Fan Yang 1 , Ji‐Qiang Chen 1 , Ying Xiong 1 , Jian‐Hua Xie 1 , Qi‐Lin Zhou 1
Affiliation
|
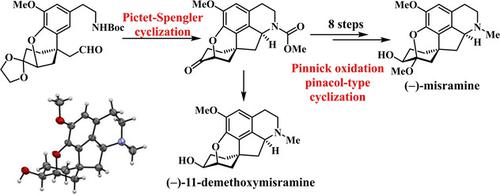
An enantioselective total synthesis of pentacyclic proaporphine alkaloid (−)‐misramine has been achieved. The synthetic route features a Pictet‐Spengler cyclization to construct the fused B and C rings and a sequence of Baeyer‐Villiger oxidation, Pinnick oxidation, and pinacol‐type cyclization to install the α‐hydroxy ketal moiety. The absolute configuration of the pentacyclic framework was elucidated by X‐ray single‐crystal analyses of (−)‐11‐demethoxymisramine.
中文翻译:

对映选择性全合成五环普鲁啡碱生物碱(-)-米沙明
已实现对映选择性的五环前胃啡肽生物碱(-)-misramine的合成。合成路线具有Pictet-Spengler环化作用以构建稠合的B和C环,以及一系列Baeyer-Villiger氧化,Pinnick氧化作用和Pinacol型环化作用以安装α-羟基缩酮部分。通过(-)-11-去甲氧基三聚氰胺的X射线单晶分析阐明了五环骨架的绝对构型。
更新日期:2020-11-18
中文翻译:

对映选择性全合成五环普鲁啡碱生物碱(-)-米沙明
已实现对映选择性的五环前胃啡肽生物碱(-)-misramine的合成。合成路线具有Pictet-Spengler环化作用以构建稠合的B和C环,以及一系列Baeyer-Villiger氧化,Pinnick氧化作用和Pinacol型环化作用以安装α-羟基缩酮部分。通过(-)-11-去甲氧基三聚氰胺的X射线单晶分析阐明了五环骨架的绝对构型。


























 京公网安备 11010802027423号
京公网安备 11010802027423号