当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ring opening of 2,6‐diaryl‐3,5‐diphenyl piperidine‐4‐one by acetic acid: Structural studies and Hirshfeld surface analysis of (E)‐4‐aryl‐1,3‐diphenylbut‐3‐en‐2‐ones
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-11-19 , DOI: 10.1002/jhet.4188 Gopal Vengatesh 1 , Manthiram Sundaravadivelu 1 , Shanmugam Muthusubramanian 2
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-11-19 , DOI: 10.1002/jhet.4188 Gopal Vengatesh 1 , Manthiram Sundaravadivelu 1 , Shanmugam Muthusubramanian 2
Affiliation
|
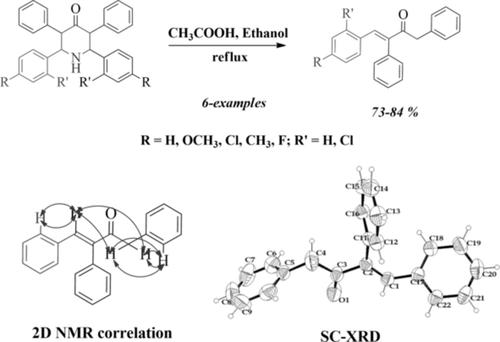
Six α,β‐unsaturated carbonyl compounds have been obtained from 2,6‐diaryl‐3,5‐diphenylpiperidine‐4‐one by the treatment with acetic acid. A plausible mechanism for the ring opening of 2,6‐diaryl‐3,5‐diphenyl piperidin‐4‐ones is proposed. The resultant (E)‐4‐aryl‐1,3‐diphenylbut‐3‐en‐2‐ones have been characterized by 1H and 13C nuclear magnetic resonance (NMR) spectral studies. One of the compounds was completely characterized by 2D NMR techniques, and structure of the compound is further proved by single‐crystal X‐ray diffraction (XRD) analysis. The crystal structure, three‐dimensional framework of crystal, and Hirshfeld surface analysis of two of the compounds have been discussed. In the crystals, molecules are mainly linked by CH···O hydrogen bonding interactions, and this interaction forms an inversion dimer with R21(6) and R21(10) ring motifs in one of the compounds. Hirshfeld surface indicates the dominance of H···H contacts on the overall surface.
中文翻译:

2,6-二芳基-3-3,5-二苯基哌啶-4-乙酸的开环:(E)-4-芳基-1,3-二苯基但是-3-en-2-en的结构研究和Hirshfeld表面分析那些
通过用乙酸处理,从2,6-二芳基-3,5-二苯基哌啶-4-中获得了六个α,β-不饱和羰基化合物。提出了2,6-二芳基-3,5-二苯基哌啶-4-酮开环的可能机理。通过1 H和13 C核磁共振(NMR)光谱研究表征了所得的(E)-4-芳基-1,3-二苯基丁-3-烯-2-酮。其中一种化合物已通过2D NMR技术进行了完全表征,并且该化合物的结构通过单晶X射线衍射(XRD)分析得到了进一步证明。讨论了两种化合物的晶体结构,晶体的三维构架和Hirshfeld表面分析。在晶体中,分子主要由C连接H···O氢键相互作用,并且该相互作用在其中一种化合物中形成具有R 2 1(6)和R 2 1(10)环基序的反相二聚体。Hirshfeld表面表示H···H接触在整个表面上的优势。
更新日期:2020-11-19
中文翻译:

2,6-二芳基-3-3,5-二苯基哌啶-4-乙酸的开环:(E)-4-芳基-1,3-二苯基但是-3-en-2-en的结构研究和Hirshfeld表面分析那些
通过用乙酸处理,从2,6-二芳基-3,5-二苯基哌啶-4-中获得了六个α,β-不饱和羰基化合物。提出了2,6-二芳基-3,5-二苯基哌啶-4-酮开环的可能机理。通过1 H和13 C核磁共振(NMR)光谱研究表征了所得的(E)-4-芳基-1,3-二苯基丁-3-烯-2-酮。其中一种化合物已通过2D NMR技术进行了完全表征,并且该化合物的结构通过单晶X射线衍射(XRD)分析得到了进一步证明。讨论了两种化合物的晶体结构,晶体的三维构架和Hirshfeld表面分析。在晶体中,分子主要由C连接H···O氢键相互作用,并且该相互作用在其中一种化合物中形成具有R 2 1(6)和R 2 1(10)环基序的反相二聚体。Hirshfeld表面表示H···H接触在整个表面上的优势。



























 京公网安备 11010802027423号
京公网安备 11010802027423号