Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2020-11-19 , DOI: 10.1016/j.jhazmat.2020.124630 Jiangang Ku , Lin Zhang , Weng Fu , Shubin Wang , Wanzhong Yin , Huihuang Chen
|
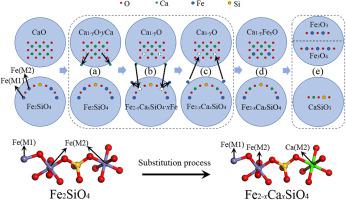
Copper slag, which contains Fe-rich fayalite (Fe2SiO4), is a valuable solid waste that warrants further research in order to recover iron. Calcium oxide (CaO) can significantly enhance iron recovery from copper slag; however, the associated mechanism has not yet been explored. In this study, we investigated the interaction between CaO and Fe2SiO4 to obtain detailed understanding of the role of CaO in enhancing iron recovery. The presence of CaO was found to accelerate the decomposition of Fe2SiO4 via an ion-exchange-like process. Specifically, CaO dissociated into Ca(II) and a Ca-deficient Ca1-yO species at high temperatures. The Fe(II) ion at the M2 site of Fe2SiO4 was substituted by the released Ca(II) ion, resulting in the formation of [(Fe(2-x)Cax)SiO4]∙xFe(II). Subsequently, the substituted Fe(II) occupied the Ca vacancy in Ca1-yO to form (Ca(1-y)Fe(II)y)O. The disproportionation of Fe(II) and the combination reaction between CaO and the SiO2 separated from Fe2SiO4 led to the generation of the final products, viz. Fe2O3, Fe3O4, and CaSiO3. This study explains the specific role of CaO in decomposing Fe2SiO4. This would not only provide theoretical guidance for iron recovery from copper slag but also present a new perspective on the recycling of valuable resources from many other smelting slags (e.g., iron slag, lead slag, and nickel slag).
中文翻译:

钙离子扩散入方铁石的机理研究:迈向铜渣可持续管理的一步
铜渣中含有富铁的铁橄榄石(Fe 2 SiO 4),是一种有价值的固体废物,需要进一步研究以回收铁。氧化钙(CaO)可以显着提高铜渣中铁的回收率;但是,尚未探索相关的机制。在这项研究中,我们调查了CaO和Fe 2 SiO 4之间的相互作用,以详细了解CaO在促进铁回收中的作用。发现CaO的存在通过类似离子交换的过程加速了Fe 2 SiO 4的分解。具体来说,CaO分解为Ca(II)和缺钙的Ca 1 - y高温下的O类。Fe 2 SiO 4的M2位处的Fe(II)离子被释放的Ca(II)离子取代,从而形成[(Fe (2- x) Ca x)SiO 4 ]∙ x Fe(II )。随后,取代的Fe(II)在Ca 1- y O中占据Ca空位,形成(Ca (1- y) Fe(II)y)O。Fe(II)的歧化以及CaO与从Fe 2 SiO 4中分离出的SiO 2的组合反应导致最终产物的生成,即。铁2 O3,Fe 3 O 4和CaSiO 3。这项研究解释了CaO在分解Fe 2 SiO 4中的特定作用。这不仅将为从铜渣中回收铁提供理论指导,而且还将为从许多其他冶炼渣(例如铁渣,铅渣和镍渣)中回收宝贵资源提供新的视角。



























 京公网安备 11010802027423号
京公网安备 11010802027423号