当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical, mechanistic, and DFT studies of amine derived diphosphines containing Ru(II)–cymene complexes with potent in vitro cytotoxic activity against HeLa and triple-negative breast cancer cells MDA-MB-231
Dalton Transactions ( IF 4 ) Pub Date : 2020-11-03 , DOI: 10.1039/d0dt02500c Juliana P da Silva 1 , Otávio Fuganti , M Gabriela Kramer , Gianella Facchin , Lucas E N Aquino , Javier Ellena , Davi F Back , Ana C S Gondim , Eduardo H S Sousa , Luiz G F Lopes , Silvane Machado , Ivelise D L Guimarães , Karen Wohnrath , Márcio P de Araujo
Dalton Transactions ( IF 4 ) Pub Date : 2020-11-03 , DOI: 10.1039/d0dt02500c Juliana P da Silva 1 , Otávio Fuganti , M Gabriela Kramer , Gianella Facchin , Lucas E N Aquino , Javier Ellena , Davi F Back , Ana C S Gondim , Eduardo H S Sousa , Luiz G F Lopes , Silvane Machado , Ivelise D L Guimarães , Karen Wohnrath , Márcio P de Araujo
Affiliation
|
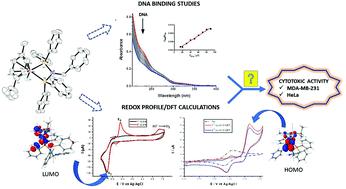
Complexes with general formula [RuCl(η6-p-cymene)(P–NR–P)]X (R = CH2Py (Py = pyridine) – [1a]+, CH2Ph (Ph = phenyl) – [1b]+, Ph – [1c] and p-tol (p-tol = p-tolyl) – [1d]+; X = PF6− or BF4−) were evaluated as cytotoxic agents against two cancer cell lines (HeLa and MDA-MB-231). All metal complexes are active in the range of concentrations tested (up to 100 μmol L−1). The IC50 (μmol L−1) values for the metal complexes are lower than that found for cisplatin. The activities are up to 6- and 15-fold higher than cisplatin for HeLa and MDA-MB-231 cancer cell lines, respectively. Studies of DNA binding and DNA cleavage were performed. DNA binding studies revealed a modest hypochromic shift in the metal complexes electronic spectra, indicating a weak interaction with Kb values in the range of 1.7 × 103–1.6 × 104. Although the cleavage tests revealed that in the dark DNA is not a biological target for these metal complexes, upon blue light irradiation they are activated causing DNA cleavage. Electrochemical studies showed the presence of two independent redox processes, one attributed to the oxidation process of Ru2+ → Ru3+ (EC process) and the other one to the reduction of Ru2+ → Ru1+, which is further reduced to Ru0 (ECE mechanism). In both processes, coupled chemical reactions were observed. DFT calculations were performed to support the electrochemical/chemical behavior of the complexes. The reactivity of complex [1b]BF4 with CH3CN was evaluated and two complexes were isolated [2b]BF4 and [3b]BF4. The complex mer-[RuCl(CH3CN)3(P–NCH2Ph–P)]BF4 ([2b]BF4) was isolated after refluxing the precursor [1b]BF4 in CH3CN. Isomerization of [2b]BF4 in CH3CN resulted in the formation of fac-[RuCl(CH3CN)3(P–NCH2Ph–P)]BF4. An attempt to isolate the fac-isomer by adding diethyl ether was unsuccessful, and the complex [3b]BF4 was observed as the major component. The complex [Ru2(μ-Cl3)(CH3CN)2(P–NCH2Ph–P)2]BF4 ([3b]BF4) proved to be very stable and can be obtained from both the mer- and the fac-isomers. The molecular structures of [1b]BF4 and [3b]BF4 were solved by single-crystal X-ray diffraction.
中文翻译:

含有 Ru(II)-cymene 复合物的胺衍生二膦的电化学、机械和 DFT 研究对 HeLa 和三阴性乳腺癌细胞 MDA-MB-231 具有有效的体外细胞毒活性
具有通式 [RuCl(η 6 - p -cymene)(P–N R –P)]X (R = CH 2 Py (Py = 吡啶) – [1a] + , CH 2 Ph (Ph = 苯基) – [1B] +中,Ph - [1C]和p -tol(p -tol = p -甲苯基) - [1D] + ; X = PF 6 -或BF 4 - )的评价为针对两种癌细胞系的细胞毒性剂(海拉和 MDA-MB-231)。所有金属络合物在测试的浓度范围内(高达 100 μmol L -1)都具有活性。IC 50金属络合物的(μmol L -1 ) 值低于发现的顺铂值。对于 HeLa 和 MDA-MB-231 癌细胞系,其活性分别比顺铂高 6 倍和 15 倍。进行了 DNA 结合和 DNA 切割的研究。DNA 结合研究揭示了金属配合物电子光谱中适度的低色移,表明与K b值在 1.7 × 10 3 –1.6 × 10 4范围内的弱相互作用. 尽管裂解试验表明在黑暗中 DNA 不是这些金属配合物的生物靶标,但在蓝光照射下它们会被激活,导致 DNA 裂解。电化学研究表明存在两个独立的氧化还原过程,一个归因于 Ru 2+ → Ru 3+的氧化过程(EC 过程),另一个归因于Ru 2+ → Ru 1+的还原,后者进一步还原为Ru 0(ECE机制)。在这两个过程中,都观察到了耦合的化学反应。进行 DFT 计算以支持配合物的电化学/化学行为。络合物[1b]BF 4与CH 3的反应性评估了 CN 并分离出两种复合物[2b]BF 4和[3b]BF 4。复杂聚体-将[RuCl(CH 3 CN)3(P-N CH 2博士-P)] BF 4([图2b] BF 4)中的溶液回流前体后,分离出[1B] BF 4在CH 3 CN。[2b]BF 4在 CH 3 CN 中的异构化导致形成fac -[RuCl(CH 3 CN) 3 (P–N CH 2 Ph –P)]BF 4. 通过添加乙醚来分离fac-异构体的尝试未成功,并且观察到复合物[3b]BF 4作为主要成分。复合物 [Ru 2 (μ-Cl 3 )(CH 3 CN) 2 (P–N CH 2 Ph –P) 2 ]BF 4 ( [3b]BF 4 ) 被证明是非常稳定的,可以从mer-和fac-异构体。[1b]BF 4和[3b]BF 4的分子结构通过单晶X射线衍射解析。
更新日期:2020-11-18
中文翻译:

含有 Ru(II)-cymene 复合物的胺衍生二膦的电化学、机械和 DFT 研究对 HeLa 和三阴性乳腺癌细胞 MDA-MB-231 具有有效的体外细胞毒活性
具有通式 [RuCl(η 6 - p -cymene)(P–N R –P)]X (R = CH 2 Py (Py = 吡啶) – [1a] + , CH 2 Ph (Ph = 苯基) – [1B] +中,Ph - [1C]和p -tol(p -tol = p -甲苯基) - [1D] + ; X = PF 6 -或BF 4 - )的评价为针对两种癌细胞系的细胞毒性剂(海拉和 MDA-MB-231)。所有金属络合物在测试的浓度范围内(高达 100 μmol L -1)都具有活性。IC 50金属络合物的(μmol L -1 ) 值低于发现的顺铂值。对于 HeLa 和 MDA-MB-231 癌细胞系,其活性分别比顺铂高 6 倍和 15 倍。进行了 DNA 结合和 DNA 切割的研究。DNA 结合研究揭示了金属配合物电子光谱中适度的低色移,表明与K b值在 1.7 × 10 3 –1.6 × 10 4范围内的弱相互作用. 尽管裂解试验表明在黑暗中 DNA 不是这些金属配合物的生物靶标,但在蓝光照射下它们会被激活,导致 DNA 裂解。电化学研究表明存在两个独立的氧化还原过程,一个归因于 Ru 2+ → Ru 3+的氧化过程(EC 过程),另一个归因于Ru 2+ → Ru 1+的还原,后者进一步还原为Ru 0(ECE机制)。在这两个过程中,都观察到了耦合的化学反应。进行 DFT 计算以支持配合物的电化学/化学行为。络合物[1b]BF 4与CH 3的反应性评估了 CN 并分离出两种复合物[2b]BF 4和[3b]BF 4。复杂聚体-将[RuCl(CH 3 CN)3(P-N CH 2博士-P)] BF 4([图2b] BF 4)中的溶液回流前体后,分离出[1B] BF 4在CH 3 CN。[2b]BF 4在 CH 3 CN 中的异构化导致形成fac -[RuCl(CH 3 CN) 3 (P–N CH 2 Ph –P)]BF 4. 通过添加乙醚来分离fac-异构体的尝试未成功,并且观察到复合物[3b]BF 4作为主要成分。复合物 [Ru 2 (μ-Cl 3 )(CH 3 CN) 2 (P–N CH 2 Ph –P) 2 ]BF 4 ( [3b]BF 4 ) 被证明是非常稳定的,可以从mer-和fac-异构体。[1b]BF 4和[3b]BF 4的分子结构通过单晶X射线衍射解析。



























 京公网安备 11010802027423号
京公网安备 11010802027423号