Mini-Reviews in Medicinal Chemistry ( IF 3.8 ) Pub Date : 2020-09-30 , DOI: 10.2174/1389557520666200619121003 Jiangming Wang 1 , Silei Lu 1 , Ruilong Sheng 2 , Junting Fan 3 , Wenhui Wu 1 , Ruihua Guo 1
|
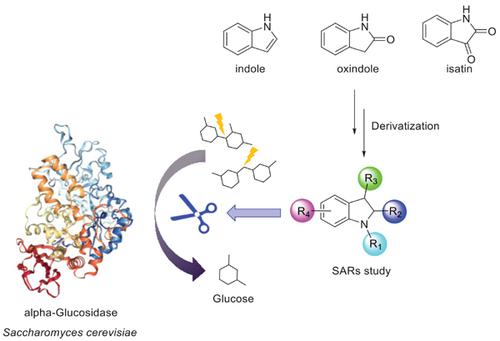
α-Glucosidase plays an important role in carbohydrate metabolism and is an attractive drug target for the treatment of diabetes, obesity and other related complications. Currently, acarbose, miglitol and voglibose have been approved by the FDA for the treatment of diabetes by oral α-glucosidase inhibitors. With the development of anti-diabetic drugs, the emergence of novel drugs with various chemotypes has overshadowed α-glucosidase inhibitors. Since the 1990s, the FDA has not approved new chemical entities against α-glucosidase, which has resulted in restricted clinical medication. Nevertheless, this type of inhibitors possess several unparalleled advantages over other drugs, especially mild side effects (non-systemic gastrointestinal side effects and occasional allergic reactions). Additionally, α-glucosidase inhibitors for monotherapy or in combination with other drugs have been proved to be a feasible approach for the treatment of diabetes. In the last decade, the discovery of natural or synthetic indole derivatives possessing the inhibitory activity of α-glucosidase has received great attention. Herein, we have summarized indoles as inhibitors of α-glucosidase activity, their mechanism of action, synthetic methodologies and structure-activity relationships. Moreover, we have compared the inhibitory potencies of all compounds under their corresponding positive control as well as oral absorption in silico evaluated by tPSA. This review will provide a medium on which future drug design and development for the treatment of diabetes may be modeled as many drug candidates with present great potential as effective anti-diabetic chemotherapy.
中文翻译:

天然和合成的吲哚衍生支架作为α-葡萄糖苷酶抑制剂的结构-活性关系:迷你审查。
α-葡萄糖苷酶在碳水化合物代谢中起重要作用,并且是治疗糖尿病,肥胖症和其他相关并发症的有吸引力的药物靶标。当前,阿卡波糖,米格列醇和伏格列波糖已被FDA批准通过口服α-葡萄糖苷酶抑制剂治疗糖尿病。随着抗糖尿病药的发展,具有各种化学型的新药的出现使α-葡萄糖苷酶抑制剂的前景黯淡。自1990年代以来,FDA一直未批准针对α-葡萄糖苷酶的新化学实体,这导致临床用药受到限制。然而,这种抑制剂与其他药物相比具有一些无与伦比的优势,尤其是轻度的副作用(非全身性胃肠道副作用和偶发的过敏反应)。另外,已经证明,将α-葡萄糖苷酶抑制剂用于单药治疗或与其他药物联合使用是治疗糖尿病的可行方法。在最近的十年中,具有α-葡萄糖苷酶抑制活性的天然或合成吲哚衍生物的发现受到了极大的关注。本文中,我们总结了吲哚作为α-葡萄糖苷酶活性的抑制剂,它们的作用机理,合成方法和构效关系。此外,我们已经比较了所有化合物在其相应的阳性对照下的抑制能力以及通过tPSA评估的口服硅吸收。这篇综述将提供一种媒介,可以用来模拟未来治疗糖尿病的药物设计和开发,因为许多候选药物具有作为有效的抗糖尿病化学疗法的巨大潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号