当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pathway Alteration of Water Splitting via Oxygen Vacancy Formation on Anatase Titanium Dioxide in Photothermal Catalysis
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-11-17 , DOI: 10.1021/acs.jpcc.0c07117 Zheng Li 1 , Xuhan Zhang 1 , Li Zhang 1 , Chenyu Xu 2 , Yanwei Zhang 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-11-17 , DOI: 10.1021/acs.jpcc.0c07117 Zheng Li 1 , Xuhan Zhang 1 , Li Zhang 1 , Chenyu Xu 2 , Yanwei Zhang 1
Affiliation
|
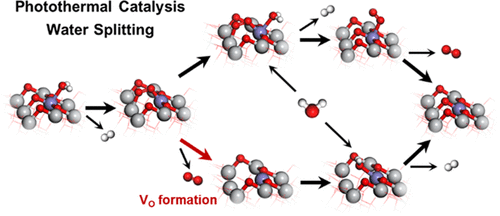
Solar-induced photothermal catalysis of water splitting is a promising method to produce hydrogen and could be promoted by surface defects, especially oxygen vacancies on the surfaces of metal oxides. The interaction between water and surface is likely to lead to the formation of oxygen vacancies and alters the pathway of overall water splitting. The complete free energy profiles of the two different pathways on the anatase TiO2(101) surface are calculated based on density functional theory, and hydrogen evolution is found to be the rate-determining step. The doping of Fe and Cu both reduces the energy barriers of oxygen vacancy formation but has different effects on hydrogen evolution on oxygen vacancies. The balance between the formation and reactivity of surface oxygen vacancies is the key factor for the pathway preference for water splitting. Therefore, the two different pathways of overall water splitting on the surfaces of metal oxides should be taken into consideration for photothermal catalyst design and screening.
中文翻译:

光热催化中锐钛矿型二氧化钛上空位形成氧分解水的途径改变
太阳诱导的水分解的光热催化是产生氢的有前途的方法,并且可以通过表面缺陷,特别是金属氧化物表面上的氧空位来促进。水与表面之间的相互作用很可能导致氧空位的形成,并改变整个水分解的途径。锐钛矿TiO 2上两个不同途径的完整自由能谱基于密度泛函理论计算(101)的表面,发现氢的释放是决定速率的步骤。Fe和Cu的掺杂都降低了氧空位形成的能垒,但对氢的空位释放有不同的影响。表面氧空位的形成和反应性之间的平衡是水分解途径偏好的关键因素。因此,在光热催化剂的设计和筛选中,应考虑金属氧化物表面上总水分解的两种不同途径。
更新日期:2020-12-03
中文翻译:

光热催化中锐钛矿型二氧化钛上空位形成氧分解水的途径改变
太阳诱导的水分解的光热催化是产生氢的有前途的方法,并且可以通过表面缺陷,特别是金属氧化物表面上的氧空位来促进。水与表面之间的相互作用很可能导致氧空位的形成,并改变整个水分解的途径。锐钛矿TiO 2上两个不同途径的完整自由能谱基于密度泛函理论计算(101)的表面,发现氢的释放是决定速率的步骤。Fe和Cu的掺杂都降低了氧空位形成的能垒,但对氢的空位释放有不同的影响。表面氧空位的形成和反应性之间的平衡是水分解途径偏好的关键因素。因此,在光热催化剂的设计和筛选中,应考虑金属氧化物表面上总水分解的两种不同途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号