当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Equilibrium Solubility Investigation and Thermodynamic Computational Modeling of Halosulfuron-methyl in a Series of Cosolvent Mixtures at a Temperature Range of 278.15–323.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-11-18 , DOI: 10.1021/acs.jced.0c00633 Guang Yang 1 , Jianqiang Zhang 1 , Chunjuan Huang 2 , Xin Song 2 , Renjie Xu 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-11-18 , DOI: 10.1021/acs.jced.0c00633 Guang Yang 1 , Jianqiang Zhang 1 , Chunjuan Huang 2 , Xin Song 2 , Renjie Xu 2
Affiliation
|
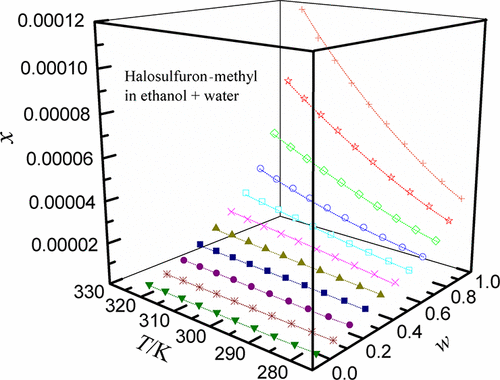
Water as an auxiliary solvent is added to various alcohol solvents (methanol, ethanol, and isopropanol) and N,N-dimethylformamide (DMF) to form a variety of liquid mixtures, which are divided into different types according to different proportions. Starting from pure water, 0.1 mass fraction of organic solvents is added at a time until the ratio of organic solvent is 1. The abovementioned liquid mixtures are used as solvents to dissolve halosulfuron-methyl at 278.15–323.15 K under 101.2 kPa by means of the isothermal static technique. The experimental solubility of halosulfuron-methyl was calculated and validated by using three various computational models, i.e., Jouyban–Acree model, modified Apelblat–Jouyban–Acree model, and van’t Hoff–Jouyban–Acree model. Considering that there are many factors that change the experimental results, this experiment mainly selected temperature and water content as two factors as the main independent variables. From the point of view of temperature, it changes from a low value to a high value, so it is obvious that the solute mole fraction also follows its footsteps. Unlike the temperature, as the organic solvent is continuously diluted by water, the solute solubility changes in the opposite direction to the water content. By controlling the variable method to ensure that the above two variables remain unchanged, DMF has the best dispersion ability for all the selected solvents to dissolve halosulfuron-methyl. The solubility maximum of 2.542 × 10–3 at 323.15 K was found in pure DMF, and four systems followed the descending order as (DMF + water) > (ethanol + water) > (methanol + water) > (isopropanol + water). The largest relative average deviation and root-mean-square deviation values were 5.70 × 10–3 and 6.90 × 10–6, respectively. For drugs with poor water solubility and dissolution rate, measuring their solubility and understanding their dissolution properties could effectively help solve the problem of nonwetting and nondiffusion. The obtained equilibrium solubility and correlation parameters of halosulfuron-methyl in studied cosolvency systems could be favorable for the industrial production, recrystallization, and purification processes of API halosulfuron-methyl.
中文翻译:

温度范围为278.15–323.15 K的一系列共溶剂混合物中卤代磺隆的平衡溶解度研究和热力学计算模型
将水作为辅助溶剂添加到各种醇溶剂(甲醇,乙醇和异丙醇)和N,N-二甲基甲酰胺(DMF)中,形成各种液体混合物,根据不同的比例将其分为不同的类型。从纯净水开始,一次添加0.1质量分数的有机溶剂,直至有机溶剂的比例为1。上述液体混合物用作溶剂,通过101.2 kPa在278.15–323.15 K下溶解卤代磺隆甲基。等温静态技术。使用三种不同的计算模型计算并验证了卤代磺隆的实验溶解度,即,Jouyban-Acree模型,改良的Apelblat-Jouyban-Acree模型和van't Hoff-Jouyban-Acree模型。考虑到影响实验结果的因素很多,本实验主要选择温度和水含量为两个因素作为主要自变量。从温度的角度来看,它从低值变为高值,因此很明显溶质摩尔分数也跟随其脚步。与温度不同,随着有机溶剂被水连续稀释,溶质的溶解度沿与水含量相反的方向变化。通过控制变量方法以确保上述两个变量保持不变,DMF对所有选定的溶剂具有最佳的溶解卤代磺隆甲基的分散能力。溶解度最大值为2.542×10在纯DMF中发现323.15 K处的–3,四个系统的降序为(DMF +水)>(乙醇+水)>(甲醇+水)>(异丙醇+水)。最大相对平均偏差和均方根偏差分别为5.70×10 –3和6.90×10 –6。对于水溶性和溶解度较差的药物,测量其溶解度并了解其溶解特性可以有效地解决不润湿和不扩散的问题。在所研究的共溶剂体系中所获得的卤代磺隆的平衡溶解度和相关参数,可能有利于API卤代磺隆的工业化生产,重结晶和纯化工艺。
更新日期:2021-01-14
中文翻译:

温度范围为278.15–323.15 K的一系列共溶剂混合物中卤代磺隆的平衡溶解度研究和热力学计算模型
将水作为辅助溶剂添加到各种醇溶剂(甲醇,乙醇和异丙醇)和N,N-二甲基甲酰胺(DMF)中,形成各种液体混合物,根据不同的比例将其分为不同的类型。从纯净水开始,一次添加0.1质量分数的有机溶剂,直至有机溶剂的比例为1。上述液体混合物用作溶剂,通过101.2 kPa在278.15–323.15 K下溶解卤代磺隆甲基。等温静态技术。使用三种不同的计算模型计算并验证了卤代磺隆的实验溶解度,即,Jouyban-Acree模型,改良的Apelblat-Jouyban-Acree模型和van't Hoff-Jouyban-Acree模型。考虑到影响实验结果的因素很多,本实验主要选择温度和水含量为两个因素作为主要自变量。从温度的角度来看,它从低值变为高值,因此很明显溶质摩尔分数也跟随其脚步。与温度不同,随着有机溶剂被水连续稀释,溶质的溶解度沿与水含量相反的方向变化。通过控制变量方法以确保上述两个变量保持不变,DMF对所有选定的溶剂具有最佳的溶解卤代磺隆甲基的分散能力。溶解度最大值为2.542×10在纯DMF中发现323.15 K处的–3,四个系统的降序为(DMF +水)>(乙醇+水)>(甲醇+水)>(异丙醇+水)。最大相对平均偏差和均方根偏差分别为5.70×10 –3和6.90×10 –6。对于水溶性和溶解度较差的药物,测量其溶解度并了解其溶解特性可以有效地解决不润湿和不扩散的问题。在所研究的共溶剂体系中所获得的卤代磺隆的平衡溶解度和相关参数,可能有利于API卤代磺隆的工业化生产,重结晶和纯化工艺。



























 京公网安备 11010802027423号
京公网安备 11010802027423号