当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multienzyme Cellulose Films as Sustainable and Self-Degradable Hydrogen Peroxide-Producing Material
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-11-17 , DOI: 10.1021/acs.biomac.0c01393 Davide Califano 1 , Marco A S Kadowaki 2 , Vincenzo Calabrese 1 , Rolf Alexander Prade 3 , Davide Mattia 4 , Karen J Edler 1 , Igor Polikarpov 2 , Janet L Scott 1
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-11-17 , DOI: 10.1021/acs.biomac.0c01393 Davide Califano 1 , Marco A S Kadowaki 2 , Vincenzo Calabrese 1 , Rolf Alexander Prade 3 , Davide Mattia 4 , Karen J Edler 1 , Igor Polikarpov 2 , Janet L Scott 1
Affiliation
|
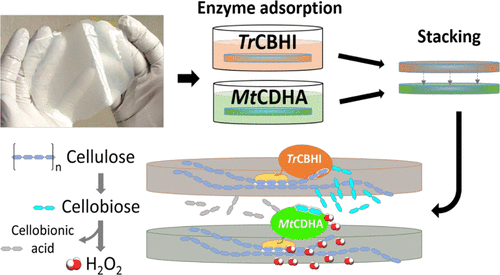
The use of hydrogen peroxide-releasing enzymes as a component to produce alternative and sustainable antimicrobial materials has aroused interest in the scientific community. However, the preparation of such materials requires an effective enzyme binding method that often involves the use of expensive and toxic chemicals. Here, we describe the development of an enzyme-based hydrogen peroxide-producing regenerated cellulose film (RCF) in which a cellobiohydrolase (TrCBHI) and a cellobiose dehydrogenase (MtCDHA) were efficiently adsorbed, 90.38 ± 2.2 and 82.40 ± 5.7%, respectively, without making use of cross-linkers. The enzyme adsorption kinetics and binding isotherm experiments showed high affinity of the proteins possessing cellulose-binding modules for RCF, suggesting that binding on regenerated cellulose via specific interactions can be an alternative method for enzyme immobilization. Resistance to compression and porosity at a micrometer scale were found to be tunable by changing cellulose concentration prior to film regeneration. The self-degradation process, triggered by stacking TrCBHI and MtCDHA (previously immobilized onto separate RCF), produced 0.15 nmol/min·cm2 of H2O2. Moreover, the production of H2O2 was sustained for at least 24 h reaching a concentration of ∼2 mM. The activity of MtCDHA immobilized on RCF was not affected by reuse for at least 3 days (1 cycle/day), suggesting that no significant enzyme leakage occurred in that timeframe. In the material herein designed, cellulose (regenerated from a 1-ethyl-3-methylimidazolium acetate/dimethyl sulfoxide (DMSO) solution) serves both as support and substrate for the immobilized enzymes. The sequential reaction led to the production of H2O2 at a micromolar–millimolar level revealing the potential use of the material as a self-degradable antimicrobial agent.
中文翻译:

多酶纤维素膜作为可持续的和可自降解的过氧化氢生产材料
使用过氧化氢释放酶作为生产替代性和可持续性抗菌材料的成分已引起了科学界的兴趣。但是,此类材料的制备需要有效的酶结合方法,该方法通常涉及使用昂贵且有毒的化学物质。在这里,我们描述了一种基于酶的产生过氧化氢的再生纤维素膜(RCF)的发展,其中纤维二糖水解酶(Tr CBHI)和纤维二糖脱氢酶(MtCDHA)被有效吸附,分别为90.38±2.2和82.40±5.7%,而无需使用交联剂。酶的吸附动力学和结合等温线实验表明,具有纤维素结合模块的蛋白质对RCF的亲和力高,这表明通过特异性相互作用在再生纤维素上的结合可以成为酶固定的另一种方法。发现在膜再生之前通过改变纤维素浓度可以调节微米级的抗压性和孔隙率。通过将Tr CBHI和Mt CDHA(先前固定在单独的RCF上)堆叠而触发的自降解过程产生了0.15 nmol / min·cm 2的H 2 O 2。。此外,H 2 O 2的产生持续至少24小时,达到约2 mM的浓度。固定在RCF上的Mt CDHA的活性在至少3天(1个周期/天)内不受重复使用的影响,表明在该时间段内未发生明显的酶泄漏。在本文设计的材料中,纤维素(由乙酸1-乙基-3-甲基咪唑鎓/二甲基亚砜(DMSO)溶液再生)既用作固定酶的载体,又用作底物。顺序反应导致H 2 O 2的生成量为微摩尔-毫摩尔水平,表明该材料可作为自降解抗菌剂的潜在用途。
更新日期:2020-12-14
中文翻译:

多酶纤维素膜作为可持续的和可自降解的过氧化氢生产材料
使用过氧化氢释放酶作为生产替代性和可持续性抗菌材料的成分已引起了科学界的兴趣。但是,此类材料的制备需要有效的酶结合方法,该方法通常涉及使用昂贵且有毒的化学物质。在这里,我们描述了一种基于酶的产生过氧化氢的再生纤维素膜(RCF)的发展,其中纤维二糖水解酶(Tr CBHI)和纤维二糖脱氢酶(MtCDHA)被有效吸附,分别为90.38±2.2和82.40±5.7%,而无需使用交联剂。酶的吸附动力学和结合等温线实验表明,具有纤维素结合模块的蛋白质对RCF的亲和力高,这表明通过特异性相互作用在再生纤维素上的结合可以成为酶固定的另一种方法。发现在膜再生之前通过改变纤维素浓度可以调节微米级的抗压性和孔隙率。通过将Tr CBHI和Mt CDHA(先前固定在单独的RCF上)堆叠而触发的自降解过程产生了0.15 nmol / min·cm 2的H 2 O 2。。此外,H 2 O 2的产生持续至少24小时,达到约2 mM的浓度。固定在RCF上的Mt CDHA的活性在至少3天(1个周期/天)内不受重复使用的影响,表明在该时间段内未发生明显的酶泄漏。在本文设计的材料中,纤维素(由乙酸1-乙基-3-甲基咪唑鎓/二甲基亚砜(DMSO)溶液再生)既用作固定酶的载体,又用作底物。顺序反应导致H 2 O 2的生成量为微摩尔-毫摩尔水平,表明该材料可作为自降解抗菌剂的潜在用途。



























 京公网安备 11010802027423号
京公网安备 11010802027423号