Cell Reports Physical Science ( IF 8.9 ) Pub Date : 2020-11-18 , DOI: 10.1016/j.xcrp.2020.100255 Hangjia Shen , Longhai Pan , Tiju Thomas , Jiacheng Wang , Xuyun Guo , Ye Zhu , Kan Luo , Shiyu Du , Haichuan Guo , Graham J. Hutchings , J. Paul Attfield , Minghui Yang
|
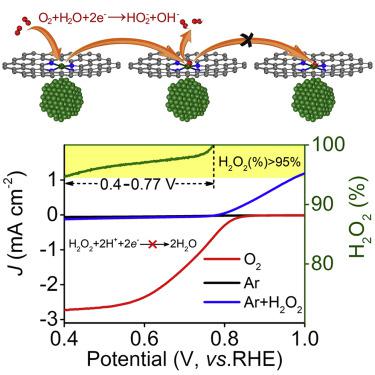
Hydrogen peroxide is a widely used industrial oxidant, the large-scale production of which continues to be done by an indirect process. Direct electrosynthesis of hydrogen peroxide from aerial oxygen and water is a sustainable alternative, but this remains challenging because hydrogen peroxide is highly reactive and robust catalysts are vital. Here, we report direct and continuous electrosynthesis of hydrogen peroxide under alkaline conditions using a nitrogen-doped-carbon-supported nickel catalyst. Both experiment and theoretical calculations confirm that the existence of nickel particles suppresses the further reduction of hydrogen peroxide on Ni-N-C matrix. In air-saturated 0.1 M potassium hydroxide, the energy-efficient non-precious metal electrocatalyst exhibits a consistent Faraday efficiency over 95% at a steady rate of hydrogen peroxide production (15.1 mmol min−1 gcat−1) for 100 h. This sustainable, efficient, and safe process is an important step toward continuous production of hydrogen peroxide.
中文翻译:

氮掺杂碳负载镍上过氧化氢的选择性和连续电合成
过氧化氢是一种广泛使用的工业氧化剂,其大规模生产继续通过间接方法进行。由空气中的氧气和水直接电合成过氧化氢是一种可持续的替代方法,但这仍然具有挑战性,因为过氧化氢具有高反应活性,而坚固的催化剂至关重要。在这里,我们报告了在碱性条件下使用氮掺杂碳负载的镍催化剂直接和连续电合成过氧化氢。实验和理论计算均证实,镍颗粒的存在抑制了Ni-NC基体上过氧化氢的进一步还原。在空气饱和的0.1 M氢氧化钾中,-1 gcat -1)100小时。这种可持续,高效和安全的过程是连续生产过氧化氢的重要一步。


























 京公网安备 11010802027423号
京公网安备 11010802027423号