当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effective electrode design and the reaction mechanism for electrochemical promotion of ammonia synthesis using Fe-based electrode catalysts
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2020-11-3 , DOI: 10.1039/d0se01385d Chien-I. Li 1, 2, 3, 4, 5 , Hiroki Matsuo 1, 2, 3, 4, 5 , Junichiro Otomo 1, 2, 3, 4, 5
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2020-11-3 , DOI: 10.1039/d0se01385d Chien-I. Li 1, 2, 3, 4, 5 , Hiroki Matsuo 1, 2, 3, 4, 5 , Junichiro Otomo 1, 2, 3, 4, 5
Affiliation
|
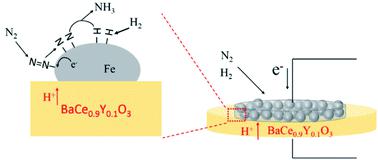
The electrochemical promotion of ammonia formation on Fe-based electrode catalysts is investigated using proton-conducting-electrolyte-supported cells of H2–Ar, Pt|BaCe0.9Y0.1O3 (BCY)| Fe-based catalysts, H2–N2 at temperatures between 550 °C and 600 °C, and ambient pressure. To clarify the reaction mechanism, the ammonia formation rate is examined using two cathodes: (I) a porous pure Fe electrode with a shorter triple phase boundary (TPB) length and (II) a cermet electrode consisting of Fe–BCY (or W–Fe–BCY) with a longer TPB length. Using the different electrode structures, we investigate the effects of cathodic polarization, hydrogen partial pressure, and electrode materials. The porous pure Fe electrode shows better performance than the Fe–BCY cermet electrode, which suggests that the ammonia formation is accelerated by the electrochemical promotion of catalysis (EPOC) effect on the Fe surface rather than the charge-transfer reaction at the TPB. The electrochemical promotion is governed by a dissociative mechanism, i.e., acceleration of direct N2 bond dissociation with cathodic polarization on the Fe surface, with a smaller contribution by a proton-assisted associative mechanism at the TPB. These findings indicate that the porous pure Fe electrode is more effective for ammonia formation than the (W–)Fe–BCY cermet electrode. Despite the relatively short TPB length, the porous pure Fe cathode achieves a very high ammonia formation rate of 1.4 × 10−8 mol cm−2 s−1 (450 μg h−1 mg−1) under appropriate conditions. This significant result suggests that the effective double layer spreads widely on the Fe electrode surface. Using the identified reaction mechanism, we discuss key processes for improving ammonia formation.
中文翻译:

铁基电极催化剂对氨合成电化学促进的有效电极设计和反应机理
使用质子导电电解质负载的H 2 -Ar,Pt | BaCe 0.9 Y 0.1 O 3(BCY)|电解质,研究了铁基电极催化剂上氨形成的电化学促进作用。铁基催化剂,H 2 -N 2在550°C和600°C之间的温度和环境压力下。为了阐明反应机理,使用两个阴极检查了氨的形成速率:(I)具有较短三相边界(TPB)长度的多孔纯铁电极,以及(II)由Fe–BCY(或W–)组成的金属陶瓷电极Fe-BCY)具有更长的TPB长度。使用不同的电极结构,我们研究了阴极极化,氢分压和电极材料的影响。多孔纯铁电极表现出比Fe-BCY金属陶瓷电极更好的性能,这表明氨的形成是通过对Fe表面的电化学催化作用(EPOC)而不是TPB上的电荷转移反应来促进的。电化学促进受解离机制控制,即,直接的N 2键解离与Fe表面的阴极极化的加速,由TPB处的质子辅助缔合机制贡献较小。这些发现表明,多孔的纯铁电极比(W-)Fe-BCY金属陶瓷电极对氨的形成更有效。尽管TPB长度相对较短,但多孔的纯铁阴极仍实现了1.4×10 -8 mol cm -2 s -1(450μgh -1 mg -1)的极高氨生成速率)在适当的条件下。该重要结果表明有效的双层在Fe电极表面上广泛铺展。使用确定的反应机理,我们讨论了改善氨生成的关键过程。
更新日期:2020-12-17
中文翻译:

铁基电极催化剂对氨合成电化学促进的有效电极设计和反应机理
使用质子导电电解质负载的H 2 -Ar,Pt | BaCe 0.9 Y 0.1 O 3(BCY)|电解质,研究了铁基电极催化剂上氨形成的电化学促进作用。铁基催化剂,H 2 -N 2在550°C和600°C之间的温度和环境压力下。为了阐明反应机理,使用两个阴极检查了氨的形成速率:(I)具有较短三相边界(TPB)长度的多孔纯铁电极,以及(II)由Fe–BCY(或W–)组成的金属陶瓷电极Fe-BCY)具有更长的TPB长度。使用不同的电极结构,我们研究了阴极极化,氢分压和电极材料的影响。多孔纯铁电极表现出比Fe-BCY金属陶瓷电极更好的性能,这表明氨的形成是通过对Fe表面的电化学催化作用(EPOC)而不是TPB上的电荷转移反应来促进的。电化学促进受解离机制控制,即,直接的N 2键解离与Fe表面的阴极极化的加速,由TPB处的质子辅助缔合机制贡献较小。这些发现表明,多孔的纯铁电极比(W-)Fe-BCY金属陶瓷电极对氨的形成更有效。尽管TPB长度相对较短,但多孔的纯铁阴极仍实现了1.4×10 -8 mol cm -2 s -1(450μgh -1 mg -1)的极高氨生成速率)在适当的条件下。该重要结果表明有效的双层在Fe电极表面上广泛铺展。使用确定的反应机理,我们讨论了改善氨生成的关键过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号