当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insight into the chemoselective aromatic vs. side-chain hydroxylation of alkylaromatics with H2O2 catalyzed by a non-heme imine-based iron complex
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020-11-03 , DOI: 10.1039/d0cy01868f Barbara Ticconi 1, 2, 3, 4, 5 , Giorgio Capocasa 1, 2, 3, 4, 5 , Andrea Cerrato 1, 2, 3, 4, 5 , Stefano Di Stefano 1, 2, 3, 4, 5 , Andrea Lapi 1, 2, 3, 4, 5 , Beatrice Marincioni 1, 2, 3, 4, 5 , Giorgio Olivo 6, 7, 8, 9 , Osvaldo Lanzalunga 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020-11-03 , DOI: 10.1039/d0cy01868f Barbara Ticconi 1, 2, 3, 4, 5 , Giorgio Capocasa 1, 2, 3, 4, 5 , Andrea Cerrato 1, 2, 3, 4, 5 , Stefano Di Stefano 1, 2, 3, 4, 5 , Andrea Lapi 1, 2, 3, 4, 5 , Beatrice Marincioni 1, 2, 3, 4, 5 , Giorgio Olivo 6, 7, 8, 9 , Osvaldo Lanzalunga 1, 2, 3, 4, 5
Affiliation
|
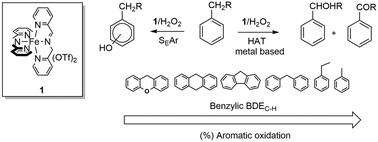
The oxidation of a series of alkylaromatic compounds with H2O2 catalyzed by an imine-based non-heme iron complex prepared in situ by reaction of 2-picolylaldehyde, 2-picolylamine, and Fe(OTf)2 in a 2 : 2 : 1 ratio leads to a marked chemoselectivity for aromatic ring hydroxylation over side-chain oxidation. This selectivity is herein investigated in detail. Side-chain/ring oxygenated product ratio was found to increase upon decreasing the bond dissociation energy (BDE) of the benzylic C–H bond in line with expectation. Evidence for competitive reactions leading either to aromatic hydroxylation via electrophilic aromatic substitution or side-chain oxidation via benzylic hydrogen atom abstraction, promoted by a metal-based oxidant, has been provided by kinetic isotope effect analysis.
中文翻译:

非血红素亚胺基铁络合物催化的过氧化氢催化烷基芳香族化合物的化学选择性芳香族和侧链羟基化
亚胺基非血红素铁配合物在2:2:2的条件下与2-picolylaldehyde,2-picolylamine和Fe(OTf)2反应原位制备的亚胺基非血红素铁配合物催化H 2 O 2氧化一系列烷基芳族化合物。1的比率导致芳环羟基化比侧链氧化具有明显的化学选择性。本文将详细研究这种选择性。发现降低苄基CH键的离解能(BDE)后,侧链/环氧化产物的比例会增加,符合预期。证据为竞争性反应导致要么芳族羟基化通过亲电芳香取代基或侧链氧化经由 动力学同位素效应分析已提供了基于金属的氧化剂促进的苄基氢原子提取。
更新日期:2020-11-17
中文翻译:

非血红素亚胺基铁络合物催化的过氧化氢催化烷基芳香族化合物的化学选择性芳香族和侧链羟基化
亚胺基非血红素铁配合物在2:2:2的条件下与2-picolylaldehyde,2-picolylamine和Fe(OTf)2反应原位制备的亚胺基非血红素铁配合物催化H 2 O 2氧化一系列烷基芳族化合物。1的比率导致芳环羟基化比侧链氧化具有明显的化学选择性。本文将详细研究这种选择性。发现降低苄基CH键的离解能(BDE)后,侧链/环氧化产物的比例会增加,符合预期。证据为竞争性反应导致要么芳族羟基化通过亲电芳香取代基或侧链氧化经由 动力学同位素效应分析已提供了基于金属的氧化剂促进的苄基氢原子提取。


























 京公网安备 11010802027423号
京公网安备 11010802027423号