当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Decarboxylative Halogenation of Organic Compounds
Chemical Reviews ( IF 62.1 ) Pub Date : 2020-11-17 , DOI: 10.1021/acs.chemrev.0c00813 Andrii Varenikov 1 , Evgeny Shapiro 1 , Mark Gandelman 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2020-11-17 , DOI: 10.1021/acs.chemrev.0c00813 Andrii Varenikov 1 , Evgeny Shapiro 1 , Mark Gandelman 1
Affiliation
|
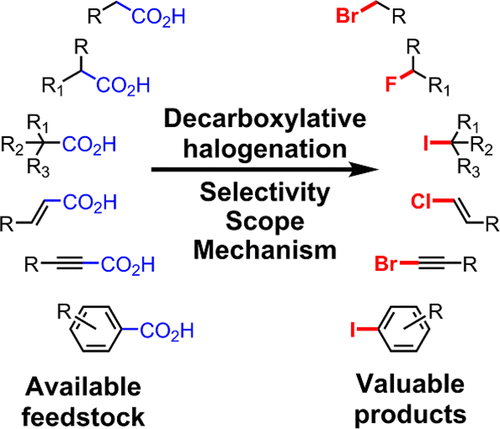
Decarboxylative halogenation, or halodecarboxylation, represents one of the fundamental key methods for the synthesis of ubiquitous organic halides. The method is based on conversion of carboxylic acids to the corresponding organic halides via selective cleavage of a carbon–carbon bond between the skeleton of the molecule and the carboxylic group and the liberation of carbon dioxide. In this review, we discuss and analyze major approaches for the conversion of alkanoic, alkenoic, acetylenic, and (hetero)aromatic acids to the corresponding alkyl, alkenyl, alkynyl, and (hetero)aryl halides. These methods include the preparation of families of valuable organic iodides, bromides, chlorides, and fluorides. The historic and modern methods for halodecarboxylation reactions are broadly discussed, including analysis of their advantages and drawbacks. We critically address the features, reaction selectivity, substrate scopes, and limitations of the approaches. In the available cases, mechanistic details of the reactions are presented, and the generality and uniqueness of the different mechanistic pathways are highlighted. The challenges, opportunities, and future directions in the field of decarboxylative halogenation are provided.
中文翻译:

有机化合物的脱羧卤化
脱羧卤化或卤代脱羧是合成普遍存在的有机卤化物的基本关键方法之一。该方法基于通过选择性断裂分子骨架和羧基之间的碳-碳键并释放二氧化碳,将羧酸转化为相应的有机卤化物。在这篇综述中,我们讨论并分析了将链烷酸、链烯酸、炔酸和(杂)芳香酸转化为相应的烷基、烯基、炔基和(杂)芳基卤化物的主要方法。这些方法包括制备有价值的有机碘化物、溴化物、氯化物和氟化物家族。广泛讨论了卤代脱羧反应的历史和现代方法,包括分析其优点和缺点。我们批判性地解决了方法的特征、反应选择性、底物范围和局限性。在现有的案例中,介绍了反应的机制细节,并强调了不同机制途径的普遍性和独特性。提供了脱羧卤化领域的挑战、机遇和未来方向。
更新日期:2021-01-13
中文翻译:

有机化合物的脱羧卤化
脱羧卤化或卤代脱羧是合成普遍存在的有机卤化物的基本关键方法之一。该方法基于通过选择性断裂分子骨架和羧基之间的碳-碳键并释放二氧化碳,将羧酸转化为相应的有机卤化物。在这篇综述中,我们讨论并分析了将链烷酸、链烯酸、炔酸和(杂)芳香酸转化为相应的烷基、烯基、炔基和(杂)芳基卤化物的主要方法。这些方法包括制备有价值的有机碘化物、溴化物、氯化物和氟化物家族。广泛讨论了卤代脱羧反应的历史和现代方法,包括分析其优点和缺点。我们批判性地解决了方法的特征、反应选择性、底物范围和局限性。在现有的案例中,介绍了反应的机制细节,并强调了不同机制途径的普遍性和独特性。提供了脱羧卤化领域的挑战、机遇和未来方向。


























 京公网安备 11010802027423号
京公网安备 11010802027423号