当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enzyme Immunoassay-Based Platform for Accurate Detection of Serum Pathological α-Synuclein in Parkinson’s Disease Patients
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-11-16 , DOI: 10.1021/acschemneuro.0c00461 Benjamin W Schlichtmann 1, 2 , Naveen Kondru 3 , Monica M Hepker 3 , Anumantha G Kanthasamy 2, 3 , Vellareddy Anantharam 2, 4 , Manohar John 2, 5 , Bhupal Ban 6 , Surya K Mallapragada 1, 2 , Balaji Narasimhan 1, 2
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-11-16 , DOI: 10.1021/acschemneuro.0c00461 Benjamin W Schlichtmann 1, 2 , Naveen Kondru 3 , Monica M Hepker 3 , Anumantha G Kanthasamy 2, 3 , Vellareddy Anantharam 2, 4 , Manohar John 2, 5 , Bhupal Ban 6 , Surya K Mallapragada 1, 2 , Balaji Narasimhan 1, 2
Affiliation
|
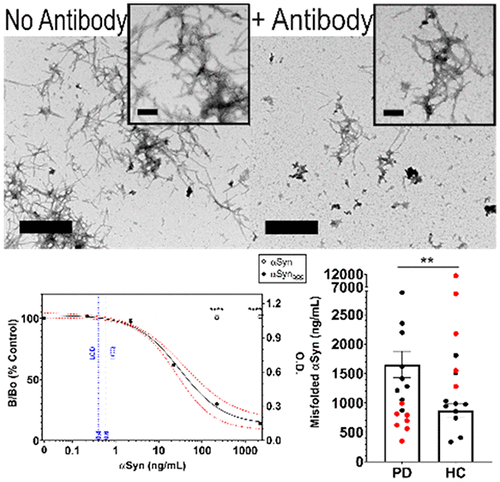
An assay for accurately diagnosing early stage Parkinson’s Disease (PD) is currently unavailable, and therefore, there is an urgent and unmet need. Such a diagnostic assay will enable prompt institution of appropriate supportive management measures to prevent rapid deterioration of disease and improve both quality of life and life expectancy of PD patients. A reliable assay platform will also be of great benefit to drug discovery and drug development in the area of PD. To this end, we describe the development of two indirect, competitive, semiquantitative enzyme immunoassays (EIAs), each employing a disparate singularly specific mouse monoclonal antibody (ssMAb) against pathological aggregates of human α-Synuclein (αSynagg), a well-established biomarker pathognomonic of PD. Our results demonstrate that these EIAs in tandem accurately discriminated between αSynagg serum concentrations from PD patients and age-matched healthy control (HC) individuals (PD = 1700 ± 220 ng/mL; HC = 870 ± 120 ng/mL with an overall sensitivity of 56%, specificity of 63%, positive predictive value of 60%, and negative predictive value of 59%). The limits of detection of αSynagg were 400 and 300 pg/mL for ssMAbs 3C5 and 5H6, respectively. These tandem EIAs have the potential to add to the repertoire of tools for earlier diagnosis of this debilitating disorder, as well as for drug development strategies.
中文翻译:

基于酶免疫分析的平台可准确检测帕金森病患者血清病理性 α-突触核蛋白
目前尚无准确诊断早期帕金森病 (PD) 的检测方法,因此存在迫切且未得到满足的需求。这种诊断测定将有助于及时采取适当的支持性管理措施,以防止疾病迅速恶化并提高帕金森病患者的生活质量和预期寿命。可靠的检测平台也将对PD领域的药物发现和药物开发大有裨益。为此,我们描述了两种间接、竞争性、半定量酶免疫分析 (EIA) 的开发,每种分析均采用不同的单一特异性小鼠单克隆抗体 (ssMAb),针对人 α-突触核蛋白 (αSyn agg) 的病理聚集体,α-突触核蛋白是一种成熟的方法。 PD 的生物标志物。我们的结果表明,这些 EIA 串联准确地区分了PD 患者和年龄匹配的健康对照 (HC) 个体的αSyn agg血清浓度(PD = 1700 ± 220 ng/mL;HC = 870 ± 120 ng/mL,总体灵敏度56%,特异性为 63%,阳性预测值为 60%,阴性预测值为 59%)。ssMAb 3C5 和 5H6 的 αSyn agg检测限分别为 400 pg/mL 和 300 pg/mL。这些串联环境影响评估有可能增加早期诊断这种衰弱性疾病以及药物开发策略的工具库。
更新日期:2020-12-16
中文翻译:

基于酶免疫分析的平台可准确检测帕金森病患者血清病理性 α-突触核蛋白
目前尚无准确诊断早期帕金森病 (PD) 的检测方法,因此存在迫切且未得到满足的需求。这种诊断测定将有助于及时采取适当的支持性管理措施,以防止疾病迅速恶化并提高帕金森病患者的生活质量和预期寿命。可靠的检测平台也将对PD领域的药物发现和药物开发大有裨益。为此,我们描述了两种间接、竞争性、半定量酶免疫分析 (EIA) 的开发,每种分析均采用不同的单一特异性小鼠单克隆抗体 (ssMAb),针对人 α-突触核蛋白 (αSyn agg) 的病理聚集体,α-突触核蛋白是一种成熟的方法。 PD 的生物标志物。我们的结果表明,这些 EIA 串联准确地区分了PD 患者和年龄匹配的健康对照 (HC) 个体的αSyn agg血清浓度(PD = 1700 ± 220 ng/mL;HC = 870 ± 120 ng/mL,总体灵敏度56%,特异性为 63%,阳性预测值为 60%,阴性预测值为 59%)。ssMAb 3C5 和 5H6 的 αSyn agg检测限分别为 400 pg/mL 和 300 pg/mL。这些串联环境影响评估有可能增加早期诊断这种衰弱性疾病以及药物开发策略的工具库。



























 京公网安备 11010802027423号
京公网安备 11010802027423号