当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
α-Synuclein Exhibits Differential Membrane Perturbation, Nucleation, and TLR2 Binding through Its Secondary Structure
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-11-16 , DOI: 10.1021/acschemneuro.0c00480 Manisha Kumari 1 , Pranita Hanpude 1 , Tushar Kanti Maiti 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-11-16 , DOI: 10.1021/acschemneuro.0c00480 Manisha Kumari 1 , Pranita Hanpude 1 , Tushar Kanti Maiti 1
Affiliation
|
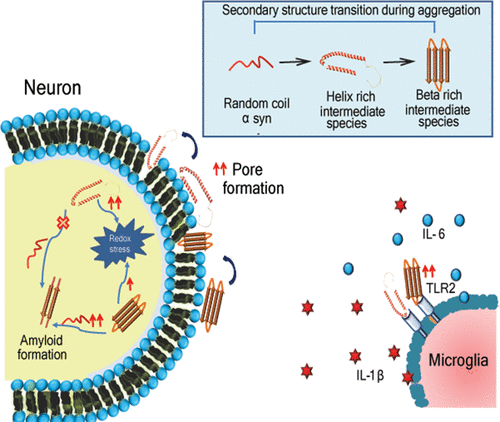
Amyloid formation drives the pathology of different neurodegenerative diseases. α-Synuclein is a natively unfolded protein that assembles itself into toxic amyloid structures, hence contributing to synucleinopathy. Its amyloid formation proceeds through various conformational intermediate stages, starting with a lag phase, followed by a rapid growth phase, and leading to beta rich fibril formation. Few studies have shown that the helix rich intermediate may be involved in fibril formation. Earlier, the helix intermediate was only studied in the membrane bound state. Despite many years of research, a precise mechanism of α-synuclein aggregation and the significance of intermediates with variable secondary structures are not well elucidated. Therefore, this study aims to understand the importance of secondary structures in α-synuclein-mediated neuronal toxicity. Our data revealed that the helix rich intermediate species exposes more of the hydrophobic surface than the beta rich intermediate species and harbors with the lipid membrane efficiently, thus contributing to the greater roughness of the cellular membrane that subsequently results in membrane disruption. It has been seen that upon internalization these species also activate the redox machinery. β-Sheet enrichment contributes to self-assemblies of monomeric α-synuclein as it binds more with the monomeric species than the helix rich species. Additionally, we also observed that the beta rich species exhibits stronger TLR2 binding than the helix rich species as well as a potentiated neuroinflammatory cascade. Taken together, our data evidently put forward that secondary structures play a differential role during amyloid formation, and targeting them can be a novel intervention strategy for neurodegenerative disease progression.
中文翻译:

α-突触核蛋白通过其二级结构表现出差异性的膜微扰,成核和TLR2绑定。
淀粉样蛋白形成驱动不同神经退行性疾病的病理。α-突触核蛋白是天然展开的蛋白,其自身组装成毒性淀粉样蛋白结构,因此导致突触核蛋白病。它的淀粉样蛋白形成通过各种构象中间阶段进行,从滞后阶段开始,然后是快速生长阶段,并导致形成富含β的原纤维。很少有研究表明富含螺旋的中间体可能与原纤维形成有关。此前,仅在膜结合状态下研究了螺旋中间体。尽管进行了多年的研究,但尚未很好地阐明α-突触核蛋白聚集的精确机理以及具有可变二级结构的中间体的重要性。因此,这项研究旨在了解二级结构在α-突触核蛋白介导的神经元毒性中的重要性。我们的数据显示,富含螺旋的中间物质比富含β的中间物质暴露更多的疏水表面,并有效地带有脂质膜,从而使细胞膜的粗糙度更高,从而导致膜破裂。已经看到,在内部化之后,这些物质也激活了氧化还原机制。β-Sheet富集有助于单体α-突触核蛋白的自组装,因为它比富含螺旋的物种与单体物种的结合更多。此外,我们还观察到,富含β的物种比富含螺旋的物种表现出更强的TLR2结合力以及增强的神经炎症级联反应。在一起
更新日期:2020-12-16
中文翻译:

α-突触核蛋白通过其二级结构表现出差异性的膜微扰,成核和TLR2绑定。
淀粉样蛋白形成驱动不同神经退行性疾病的病理。α-突触核蛋白是天然展开的蛋白,其自身组装成毒性淀粉样蛋白结构,因此导致突触核蛋白病。它的淀粉样蛋白形成通过各种构象中间阶段进行,从滞后阶段开始,然后是快速生长阶段,并导致形成富含β的原纤维。很少有研究表明富含螺旋的中间体可能与原纤维形成有关。此前,仅在膜结合状态下研究了螺旋中间体。尽管进行了多年的研究,但尚未很好地阐明α-突触核蛋白聚集的精确机理以及具有可变二级结构的中间体的重要性。因此,这项研究旨在了解二级结构在α-突触核蛋白介导的神经元毒性中的重要性。我们的数据显示,富含螺旋的中间物质比富含β的中间物质暴露更多的疏水表面,并有效地带有脂质膜,从而使细胞膜的粗糙度更高,从而导致膜破裂。已经看到,在内部化之后,这些物质也激活了氧化还原机制。β-Sheet富集有助于单体α-突触核蛋白的自组装,因为它比富含螺旋的物种与单体物种的结合更多。此外,我们还观察到,富含β的物种比富含螺旋的物种表现出更强的TLR2结合力以及增强的神经炎症级联反应。在一起

























 京公网安备 11010802027423号
京公网安备 11010802027423号