当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biochemical and Structural Investigation of GnnA in the Lipopolysaccharide Biosynthesis Pathway of Acidithiobacillus ferrooxidans
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-11-17 , DOI: 10.1021/acschembio.0c00791 Juthatip Manissorn 1 , Thassanai Sitthiyotha 2 , Jenny Rose E Montalban 3 , Surasak Chunsrivirot 2 , Peerapat Thongnuek 1 , Kittikhun Wangkanont 4
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-11-17 , DOI: 10.1021/acschembio.0c00791 Juthatip Manissorn 1 , Thassanai Sitthiyotha 2 , Jenny Rose E Montalban 3 , Surasak Chunsrivirot 2 , Peerapat Thongnuek 1 , Kittikhun Wangkanont 4
Affiliation
|
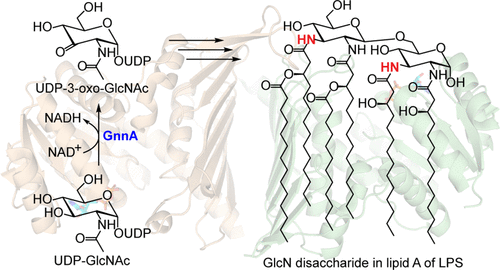
Lipopolysaccharide (LPS) is a crucial component in the outer membrane of Gram-negative bacteria that contributes to both pathogenicity as well as immunity against pathogenic bacteria. Typical LPS contains GlcN disaccharide as the core of lipid A. However, some bacteria such as Acidithiobacillus ferrooxidans and Leptospira interrogans contain GlcN3N in lipid A instead. This modification has been shown to dampen the host immune response and increase resistance to antimicrobial peptides. Therefore, investigation of the enzymes responsible for the biosynthesis of GlcN3N has promising applications in the development of vaccines, antibiotics, or usage of the enzymes in chemoenzymatic synthesis of modified LPS. Here, we describe biochemical and structural investigation of GnnA from A. ferrooxidans (AfGnnA) that is responsible for oxidation of UDP-GlcNAc, which subsequently undergoes transamination to produce UDP-GlcNAc3N as a precursor for LPS biosynthesis. AfGnnA is specific for NAD+ and UDP-GlcNAc. The crystal structures of AfGnnA in combination with molecular dynamics simulation and mutational analysis suggest the substrate recognition mode and the catalytic mechanism. K91 or H164 is a potential catalytic base in the oxidation reaction. The results will not only provide insights into the biosynthesis of unusual LPS but will also lay the foundation for development of more immunogenic vaccines, novel antibiotics, or utilization of GnnA in the synthesis of UDP-sugars or modified LPS.
中文翻译:

酸性氧化硫硫杆菌脂多糖生物合成途径中GnnA的生化和结构研究
脂多糖(LPS)是革兰氏阴性细菌外膜的重要组成部分,有助于致病性和抵抗致病性细菌的免疫力。典型的LPS含有GlcN二糖作为脂质A的核心。但是,某些细菌,如铁氧化酸硫硫杆菌和问号钩端螺旋体,则在脂质A中含有GlcN3N。已经表明这种修饰可以减弱宿主的免疫反应并增加对抗菌肽的抗性。因此,对负责GlcN3N生物合成的酶的研究在疫苗,抗生素的开发或在修饰LPS的化学合成中使用这些酶具有广阔的应用前景。在这里,我们描述了GnnA的生化和结构研究A.氧化亚铁硫杆菌(房颤GnnA),其负责UDP-GlcNAc的,其随后经历氨基转移,以产生UDP-GlcNAc3N作为LPS生物合成的前体的氧化。Af GnnA专门用于NAD +和UDP-GlcNAc。Af GnnA的晶体结构结合分子动力学模拟和突变分析表明底物识别模式和催化机制。K91或H164是氧化反应中潜在的催化碱。这些结果不仅将为不寻常的LPS的生物合成提供见识,而且还将为开发更多的免疫原性疫苗,新型抗生素或在UDP糖或修饰的LPS合成中利用GnnA奠定基础。
更新日期:2020-12-18
中文翻译:

酸性氧化硫硫杆菌脂多糖生物合成途径中GnnA的生化和结构研究
脂多糖(LPS)是革兰氏阴性细菌外膜的重要组成部分,有助于致病性和抵抗致病性细菌的免疫力。典型的LPS含有GlcN二糖作为脂质A的核心。但是,某些细菌,如铁氧化酸硫硫杆菌和问号钩端螺旋体,则在脂质A中含有GlcN3N。已经表明这种修饰可以减弱宿主的免疫反应并增加对抗菌肽的抗性。因此,对负责GlcN3N生物合成的酶的研究在疫苗,抗生素的开发或在修饰LPS的化学合成中使用这些酶具有广阔的应用前景。在这里,我们描述了GnnA的生化和结构研究A.氧化亚铁硫杆菌(房颤GnnA),其负责UDP-GlcNAc的,其随后经历氨基转移,以产生UDP-GlcNAc3N作为LPS生物合成的前体的氧化。Af GnnA专门用于NAD +和UDP-GlcNAc。Af GnnA的晶体结构结合分子动力学模拟和突变分析表明底物识别模式和催化机制。K91或H164是氧化反应中潜在的催化碱。这些结果不仅将为不寻常的LPS的生物合成提供见识,而且还将为开发更多的免疫原性疫苗,新型抗生素或在UDP糖或修饰的LPS合成中利用GnnA奠定基础。


























 京公网安备 11010802027423号
京公网安备 11010802027423号