Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ring‐opening of azetidiniums by nucleophiles. Synthesis of polysubstituted linear amines
Chirality ( IF 2 ) Pub Date : 2020-11-17 , DOI: 10.1002/chir.23280 Guillaume Masson 1 , Domingo Gomez Pardo 1 , Janine Cossy 1
Chirality ( IF 2 ) Pub Date : 2020-11-17 , DOI: 10.1002/chir.23280 Guillaume Masson 1 , Domingo Gomez Pardo 1 , Janine Cossy 1
Affiliation
|
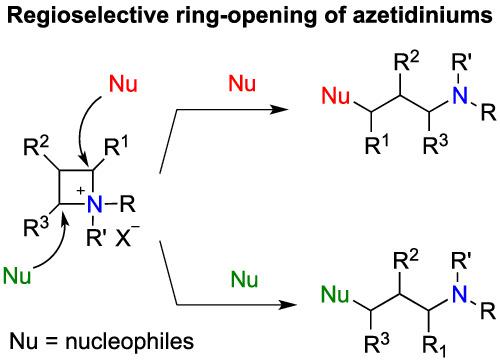
This microreview focuses on the nucleophilic ring‐opening of azetidiniums presenting various substitution patterns at C2, C3, and C4. In most cases, the nucleophilic ring‐opening occurred in a stereoselective and regioselective fashion producing functionalized linear amines. Experimental selectivities associated with Density Functional Theory (DFT) calculations have allowed a better understanding of the parameters governing the regioselectivities.
中文翻译:

亲核试剂使氮杂环丁烷开环。多取代线性胺的合成
这篇微观综述重点关注在 C2、C3 和 C4 处呈现各种取代模式的氮杂环丁烷的亲核开环。在大多数情况下,亲核开环以立体选择性和区域选择性的方式发生,产生官能化的线性胺。与密度泛函理论 (DFT) 计算相关的实验选择性可以更好地理解控制区域选择性的参数。
更新日期:2020-12-12
中文翻译:

亲核试剂使氮杂环丁烷开环。多取代线性胺的合成
这篇微观综述重点关注在 C2、C3 和 C4 处呈现各种取代模式的氮杂环丁烷的亲核开环。在大多数情况下,亲核开环以立体选择性和区域选择性的方式发生,产生官能化的线性胺。与密度泛函理论 (DFT) 计算相关的实验选择性可以更好地理解控制区域选择性的参数。



























 京公网安备 11010802027423号
京公网安备 11010802027423号