当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, structure, and photoluminescent and electroluminescent properties of zinc(II) complexes with bidentate azomethine ligands
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2020-11-17 , DOI: 10.1002/aoc.6107 Anatolii S. Burlov 1 , Valery G. Vlasenko 2 , Yurii V. Koshchienko 1 , Maxim S. Milutka 1 , Eugene I. Mal'tsev 3 , Artem V. Dmitriev 3 , Dmitry A. Lypenko 3 , Natalia V. Nekrasova 3 , Alexandra A. Kolodina 1 , Nadezhda I. Makarova 1 , Anatolii V. Metelitsa 1 , Vladimir A. Lazarenko 4 , Yan V. Zubavichus 5 , Victor N. Khrustalev 6, 7 , Dmitrii A. Garnovskii 1, 8
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2020-11-17 , DOI: 10.1002/aoc.6107 Anatolii S. Burlov 1 , Valery G. Vlasenko 2 , Yurii V. Koshchienko 1 , Maxim S. Milutka 1 , Eugene I. Mal'tsev 3 , Artem V. Dmitriev 3 , Dmitry A. Lypenko 3 , Natalia V. Nekrasova 3 , Alexandra A. Kolodina 1 , Nadezhda I. Makarova 1 , Anatolii V. Metelitsa 1 , Vladimir A. Lazarenko 4 , Yan V. Zubavichus 5 , Victor N. Khrustalev 6, 7 , Dmitrii A. Garnovskii 1, 8
Affiliation
|
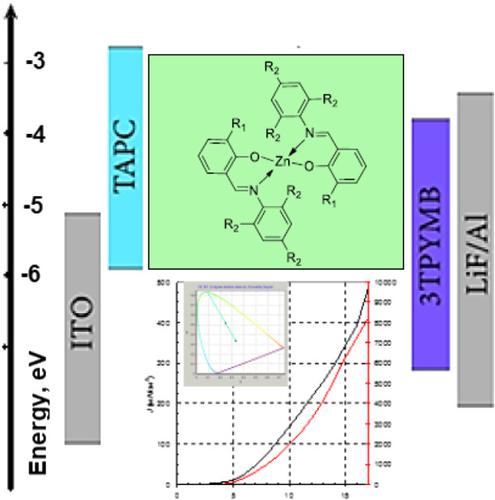
New Zn(II) complexes of bidentate Schiff base ligands were synthesized and characterized by 1H NMR, IR spectroscopy, and elemental analysis. The single‐crystal X‐ray diffraction study revealed that all compounds can be represented as mononuclear zinc(II) complexes with two bidentate organic ligands coordinated to the central metal atom by two oxygen and two nitrogen atoms. The photophysical properties of the complexes were investigated by UV–vis and photoluminescence spectroscopy in dimethyl sulfoxide (DMSO) solutions. It was found that the positions of maxima of the long‐wavelength absorption bands and photoluminescence depend strongly on the substituent in the aldehyde and amine fragments. The nature of the bands of electronic absorption spectra in DMSO solutions was interpreted using the results of quantum chemical calculations in the time‐dependent density functional theory (TD‐DFT) approximation. The light emission performance of the complexes in organic light‐emitting diodes was investigated. The electroluminescence (EL) spectra maxima of all three complexes are located in the visible range from 470 to 550 nm. The absence of additional spectral maxima or shoulders in the EL bands means that neither exciplex nor excimer states form, and the observed EL bands are due to the intrinsic radiation of the zinc(II) complexes. EQEs were measured for these three complexes. The maximum value, close to 3%, was obtained in the case of a structure with a maximal brightness of 8000 cd m−2 at 17 V.
中文翻译:

锌(II)与二齿甲亚胺配体的配合物的合成,结构以及光致发光和电致发光特性
合成了新的双齿席夫碱配体Zn(II)配合物并用1表征1 H NMR,IR光谱和元素分析。X射线单晶衍射研究表明,所有化合物都可以表示为单核锌(II)配合物,其中有两个双齿有机配体,通过两个氧和两个氮原子与中心金属原子配位。通过紫外可见光谱和光致发光光谱在二甲基亚砜(DMSO)溶液中研究了配合物的光物理性质。发现长波吸收带的最大值和光致发光的位置在很大程度上取决于醛和胺片段中的取代基。DMSO溶液中电子吸收光谱带的性质是使用时间依赖性密度泛函理论(TD-DFT)近似中的量子化学计算结果来解释的。研究了有机发光二极管中配合物的发光性能。所有三个复合物的电致发光(EL)光谱最大值位于470至550 nm的可见范围内。EL谱带中没有额外的光谱最大值或肩峰,这意味着既不形成激基态也不形成准分子态,并且观察到的EL谱带是由于锌(II)配合物的固有辐射所致。测量了这三种复合物的EQE。对于最大亮度为8000 cd m的结构,获得的最大值接近3%。EL谱带中没有额外的光谱最大值或肩峰,这意味着既不形成激基态也不形成准分子态,并且观察到的EL谱带是由于锌(II)配合物的固有辐射所致。测量了这三种复合物的EQE。对于最大亮度为8000 cd m的结构,获得的最大值接近3%。EL谱带中没有额外的光谱最大值或肩峰,这意味着既不形成激基态也不形成准分子态,并且观察到的EL谱带是由于锌(II)配合物的固有辐射所致。测量了这三种复合物的EQE。对于最大亮度为8000 cd m的结构,获得的最大值接近3%。在17 V时为−2
更新日期:2020-11-17
中文翻译:

锌(II)与二齿甲亚胺配体的配合物的合成,结构以及光致发光和电致发光特性
合成了新的双齿席夫碱配体Zn(II)配合物并用1表征1 H NMR,IR光谱和元素分析。X射线单晶衍射研究表明,所有化合物都可以表示为单核锌(II)配合物,其中有两个双齿有机配体,通过两个氧和两个氮原子与中心金属原子配位。通过紫外可见光谱和光致发光光谱在二甲基亚砜(DMSO)溶液中研究了配合物的光物理性质。发现长波吸收带的最大值和光致发光的位置在很大程度上取决于醛和胺片段中的取代基。DMSO溶液中电子吸收光谱带的性质是使用时间依赖性密度泛函理论(TD-DFT)近似中的量子化学计算结果来解释的。研究了有机发光二极管中配合物的发光性能。所有三个复合物的电致发光(EL)光谱最大值位于470至550 nm的可见范围内。EL谱带中没有额外的光谱最大值或肩峰,这意味着既不形成激基态也不形成准分子态,并且观察到的EL谱带是由于锌(II)配合物的固有辐射所致。测量了这三种复合物的EQE。对于最大亮度为8000 cd m的结构,获得的最大值接近3%。EL谱带中没有额外的光谱最大值或肩峰,这意味着既不形成激基态也不形成准分子态,并且观察到的EL谱带是由于锌(II)配合物的固有辐射所致。测量了这三种复合物的EQE。对于最大亮度为8000 cd m的结构,获得的最大值接近3%。EL谱带中没有额外的光谱最大值或肩峰,这意味着既不形成激基态也不形成准分子态,并且观察到的EL谱带是由于锌(II)配合物的固有辐射所致。测量了这三种复合物的EQE。对于最大亮度为8000 cd m的结构,获得的最大值接近3%。在17 V时为−2



























 京公网安备 11010802027423号
京公网安备 11010802027423号