当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Effect of Cation Mixing in LiNiO2 toward the Oxygen Evolution Reaction
ChemElectroChem ( IF 4 ) Pub Date : 2020-11-17 , DOI: 10.1002/celc.202001207 Yadan Ren 1 , Ryusei Yamaguchi 1 , Tomoki Uchiyama 1 , Yuki Orikasa 2 , Toshiki Watanabe 1 , Kentaro Yamamoto 1 , Toshiyuki Matsunaga 1 , Yoshinori Nishiki 3 , Shigenori Mitsushima 4, 5 , Yoshiharu Uchimoto 1
ChemElectroChem ( IF 4 ) Pub Date : 2020-11-17 , DOI: 10.1002/celc.202001207 Yadan Ren 1 , Ryusei Yamaguchi 1 , Tomoki Uchiyama 1 , Yuki Orikasa 2 , Toshiki Watanabe 1 , Kentaro Yamamoto 1 , Toshiyuki Matsunaga 1 , Yoshinori Nishiki 3 , Shigenori Mitsushima 4, 5 , Yoshiharu Uchimoto 1
Affiliation
|
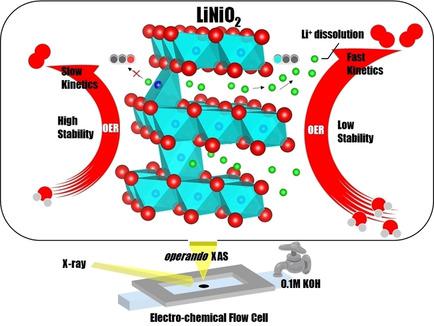
Nickel‐based oxide catalysts are widely used for the oxygen evolution reaction (OER) in alkaline water electrolysis because of their low cost and high activity. In particular, the LiNiO2 catalyst shows high activity. Therefore, to elucidate the fundamental relationship between the local structure, catalyst activity, and stability of LiNiO2, we investigated the cation mixing effect by mixing sites of lithium and nickel ions in the LiNiO2‐based catalysts. Lower degrees of cation mixing lead to higher intrinsic OER activity but lower long‐term stability. The X‐ray absorption spectra (XAS) displayed a strong hybridization state of the Ni 3d and O 2p orbitals, which is the origin of the different catalytic activity behaviors. Meanwhile, operando XAS studies combined with potentiostatic stability tests and inductively coupled plasma optical emission spectrometry (ICP‐OES) demonstrated the Li ion loss during the OER process. Thus, the instability of LiNiO2 originates from de‐intercalation of Li ions and this irreversible structure change deteriorates the performance. Hindering the lithium diffusion path by cation mixing is a useful strategy for maintaining performance. This strategy could provide a novel design principle for compatible high activity and long‐lasting catalysts by reasonable structure mediation.
中文翻译:

LiNiO2中阳离子混合对析氧反应的影响
镍基氧化物催化剂因其低成本和高活性而被广泛用于碱性水电解中的析氧反应(OER)。特别地,LiNiO 2催化剂显示出高活性。因此,为了阐明LiNiO 2的局部结构,催化剂活性和稳定性之间的基本关系,我们通过在LiNiO 2中混合锂和镍离子的位点来研究阳离子混合效应。基于催化剂。较低的阳离子混合度会导致较高的固有OER活性,但会降低长期稳定性。X射线吸收光谱(XAS)显示了Ni 3d和O 2p轨道的强杂交状态,这是不同催化活性行为的起源。同时,操作XAS研究与恒电位稳定性测试和电感耦合等离子体发射光谱(ICP-OES)相结合,证明了OER过程中锂离子的损失。因此,LiNiO 2的不稳定性源于锂离子的脱嵌作用,这种不可逆的结构变化会降低性能。通过阳离子混合阻碍锂扩散路径是保持性能的有用策略。该策略可通过合理的结构介导,为相容的高活性和长效催化剂提供新颖的设计原理。
更新日期:2021-01-04
中文翻译:

LiNiO2中阳离子混合对析氧反应的影响
镍基氧化物催化剂因其低成本和高活性而被广泛用于碱性水电解中的析氧反应(OER)。特别地,LiNiO 2催化剂显示出高活性。因此,为了阐明LiNiO 2的局部结构,催化剂活性和稳定性之间的基本关系,我们通过在LiNiO 2中混合锂和镍离子的位点来研究阳离子混合效应。基于催化剂。较低的阳离子混合度会导致较高的固有OER活性,但会降低长期稳定性。X射线吸收光谱(XAS)显示了Ni 3d和O 2p轨道的强杂交状态,这是不同催化活性行为的起源。同时,操作XAS研究与恒电位稳定性测试和电感耦合等离子体发射光谱(ICP-OES)相结合,证明了OER过程中锂离子的损失。因此,LiNiO 2的不稳定性源于锂离子的脱嵌作用,这种不可逆的结构变化会降低性能。通过阳离子混合阻碍锂扩散路径是保持性能的有用策略。该策略可通过合理的结构介导,为相容的高活性和长效催化剂提供新颖的设计原理。


























 京公网安备 11010802027423号
京公网安备 11010802027423号