当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evidence for dynamic in vivo interconversion of the conformational states of IscU during iron–sulfur cluster biosynthesis
Molecular Microbiology ( IF 3.6 ) Pub Date : 2020-11-17 , DOI: 10.1111/mmi.14646 Sakiko Sato 1 , Yumeka Matsushima 1 , Miaki Kanazawa 1 , Naoyuki Tanaka 1 , Takashi Fujishiro 1 , Kouhei Kunichika 1 , Ryosuke Nakamura 1 , Hiroaki Tomioka 2 , Kei Wada 3 , Yasuhiro Takahashi 1
Molecular Microbiology ( IF 3.6 ) Pub Date : 2020-11-17 , DOI: 10.1111/mmi.14646 Sakiko Sato 1 , Yumeka Matsushima 1 , Miaki Kanazawa 1 , Naoyuki Tanaka 1 , Takashi Fujishiro 1 , Kouhei Kunichika 1 , Ryosuke Nakamura 1 , Hiroaki Tomioka 2 , Kei Wada 3 , Yasuhiro Takahashi 1
Affiliation
|
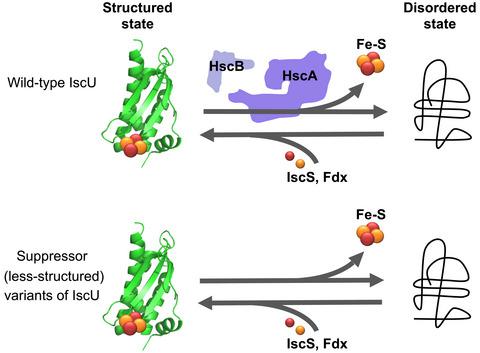
IscU is a central component of the ISC machinery and serves as a scaffold for de novo assembly of Fe–S clusters. The dedicated chaperone system composed of the Hsp70‐chaperone HscA and the J‐protein cochaperone HscB synergistically interacts with IscU and facilitates cluster transfer from IscU to recipient apo‐proteins. Here, we report that the otherwise essential roles of HscA and HscB can be bypassed in vivo by a number of single amino acid substitutions in IscU. CD spectroscopic studies of the variant IscU proteins capable of this bypass activity revealed dynamic interconversion between two conformations: the denatured (D) and the structured (S) state in the absence and presence of Zn2+, respectively, which was far more prominent than interconversion observed in wild‐type IscU. Furthermore, we found that neither the S‐shifted (more structured) variants of IscU nor the perpetually denatured variants could perform their in vivo role regardless of whether the chaperone system was present or not. The present study thus provides for the first time evidence that an in vivo D‐state of IscU exists and implies that conformational interconversion between the S‐ and D‐states of the scaffolding protein is a fundamental requirement for the assembly and transfer of the Fe–S cluster.
中文翻译:

铁硫簇生物合成过程中 IscU 构象状态动态体内相互转换的证据
IscU 是 ISC 机制的核心组件,可作为 Fe-S 簇从头组装的支架。由 Hsp70 伴侣蛋白 HscA 和 J 蛋白辅助蛋白 HscB 组成的专用伴侣系统与 IscU 协同相互作用并促进从 IscU 到受体载脂蛋白的簇转移。在这里,我们报告了 HscA 和 HscB 的其他重要作用可以通过 IscU 中的许多单个氨基酸替换在体内绕过。能够进行这种旁路活动的变体 IscU 蛋白的 CD 光谱研究揭示了两种构象之间的动态相互转换:在不存在和存在 Zn 2+的情况下,变性 (D) 和结构化 (S) 状态分别比在野生型 IscU 中观察到的相互转换要突出得多。此外,我们发现无论伴侣系统是否存在,IscU 的 S 移位(更结构化)变体和永久变性变体都不能发挥其体内作用。因此,本研究首次提供了 IscU 体内 D 态存在的证据,并暗示支架蛋白 S 态和 D 态之间的构象相互转换是 Fe– 组装和转移的基本要求。 S簇。
更新日期:2020-11-17
中文翻译:

铁硫簇生物合成过程中 IscU 构象状态动态体内相互转换的证据
IscU 是 ISC 机制的核心组件,可作为 Fe-S 簇从头组装的支架。由 Hsp70 伴侣蛋白 HscA 和 J 蛋白辅助蛋白 HscB 组成的专用伴侣系统与 IscU 协同相互作用并促进从 IscU 到受体载脂蛋白的簇转移。在这里,我们报告了 HscA 和 HscB 的其他重要作用可以通过 IscU 中的许多单个氨基酸替换在体内绕过。能够进行这种旁路活动的变体 IscU 蛋白的 CD 光谱研究揭示了两种构象之间的动态相互转换:在不存在和存在 Zn 2+的情况下,变性 (D) 和结构化 (S) 状态分别比在野生型 IscU 中观察到的相互转换要突出得多。此外,我们发现无论伴侣系统是否存在,IscU 的 S 移位(更结构化)变体和永久变性变体都不能发挥其体内作用。因此,本研究首次提供了 IscU 体内 D 态存在的证据,并暗示支架蛋白 S 态和 D 态之间的构象相互转换是 Fe– 组装和转移的基本要求。 S簇。


























 京公网安备 11010802027423号
京公网安备 11010802027423号