当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ring-opening fluorination of cyclopropylmethanols and cycloprpanecarbardehydes with diethylaminosulfur trifluoride
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-11-17 , DOI: 10.1016/j.tetlet.2020.152655 Masayuki Kirihara , You Kikkawa , Riho Nakamura , Kana Nakakura , Yasuhiro Suzuki , Yukari Muramatsu
中文翻译:

环丙基甲醇和环丙烷碳乙醛与三氟化二乙氨基硫的开环氟化反应
更新日期:2021-01-21
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-11-17 , DOI: 10.1016/j.tetlet.2020.152655 Masayuki Kirihara , You Kikkawa , Riho Nakamura , Kana Nakakura , Yasuhiro Suzuki , Yukari Muramatsu
|
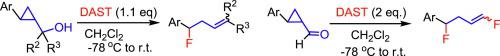
The reaction of cyclopropylmethanols bearing aromatic substituents on their cyclopropane rings with diethylaminosulfur trifluoride (DAST) effectively caused ring-opening fluorination to produce homoallylic fluorides. This reaction proceeded via a carbocation intermediate. DAST reacted with cyclopropanecarbaldehydes having electron donating aromatic substituents to cause ring-opening fluorinations with the addition of two fluorine atoms to afford 1,4-difluorobut-1-enes.
中文翻译:

环丙基甲醇和环丙烷碳乙醛与三氟化二乙氨基硫的开环氟化反应
在其环丙烷环上带有芳族取代基的环丙基甲醇与二乙基氨基三氟化硫(DAST)的反应可有效地引起开环氟化反应,从而生成均聚物氟化物。该反应通过碳正离子中间体进行。DAST与具有给电子芳族取代基的环丙烷甲醛反应,通过添加两个氟原子引起开环氟化反应,得到1,4-二氟丁-1-烯。



























 京公网安备 11010802027423号
京公网安备 11010802027423号