当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
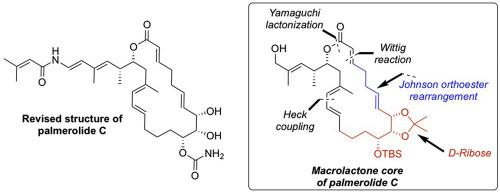
Synthesis of the macrolactone core of the revised structure of palmerolide C
Tetrahedron ( IF 2.1 ) Pub Date : 2020-11-17 , DOI: 10.1016/j.tet.2020.131768 Kannan Vaithegi , Amit B. Pawar , Kavirayani R. Prasad
中文翻译:

棕榈内酯C修饰结构的大内酯核的合成
更新日期:2020-12-16
Tetrahedron ( IF 2.1 ) Pub Date : 2020-11-17 , DOI: 10.1016/j.tet.2020.131768 Kannan Vaithegi , Amit B. Pawar , Kavirayani R. Prasad
|
Synthetic efforts towards the total synthesis of the revised structure of marine macrolide palmerolide C is reported. Synthesis of the triol unit present in the C8,C9, C10 positions was achieved from d-ribose while Johnson orthoester rearrangement and a Wittig reaction were used to construct the C1–C7 component of the macrolactone. The key macrolactone unit was assembled using intermolecular Heck coupling reaction and Yamaguchi lactonization.
中文翻译:

棕榈内酯C修饰结构的大内酯核的合成
据报道,对海洋大环内酯棕榈酰内酯C的修饰结构进行了全合成。存在于C8,C9,C10位置的三醇单元的合成是由d-核糖完成的,而Johnson Johnson原酸酯重排和Wittig反应用于构建大内酯的C1-C7组分。使用分子间的Heck偶联反应和山口内酯化组装关键的大内酯单元。


























 京公网安备 11010802027423号
京公网安备 11010802027423号