Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2020-11-17 , DOI: 10.1016/j.jhazmat.2020.124568 Xiaoting Qian , Zhouhang Gu , Qing Tang , Aimei Hong , Zhenlan Xu , Yihong Dai , Xinyun Bian , Haijin Lou , Monika Mortimer , Mohammed Baalousha , Lingxiangyu Li
|
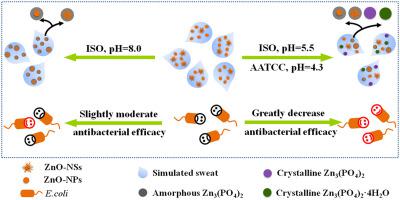
Nanoscale zinc oxide (n-ZnO) is widely used in personal care products and textiles, thus, it would likely be released into human sweat. To better evaluate the potential human health risks of n-ZnO, it is essential to understand its chemical transformations in physiological solutions, such as human sweat, and the resulting changes in the n-ZnO bioavailability. Here, two types of n-ZnO, ZnO nanoparticles (ZnO-NPs) and nanorod-based ZnO nanospheres (ZnO-NSs) were synthesized and incubated in 3 types of simulated sweat with different pH values and phosphate concentrations. The content of Zn3(PO4)2 in the transformed n-ZnO was quantified by selective dissolution of Zn3(PO4)2 in 0.35 M ammonia solution where 100% and 5.5% of Zn3(PO4)2 and ZnO were dissolved, respectively. The kinetics analysis indicated that by 24-48 h the content of Zn3(PO4)2 reached the maximum, being 15-21% at pH 8.0 and 45-70% at pH 5.5 or 4.3. Interestingly, no correlation was observed between the rate constants of Zn3(PO4)2 formation and the specific surface areas of n-ZnO, implying that chemical transformations from n-ZnO to Zn3(PO4)2 in the simulated sweat might not be simply attributed to dissolution and precipitation. Using a variety of characterization techniques, we demonstrated the formation of a ZnO-Zn3(PO4)2 core-shell structure with the shell consisting of amorphous Zn3(PO4)2 at pH 8.0 and additionally of crystalline Zn3(PO4)2 and Zn3(PO4)2•4H2O at pH 5.5 or 4.3. The phosphate-induced transformation of n-ZnO in the simulated sweat at pH 5.5 and 4.3 greatly reduced the antibacterial efficacy of n-ZnO through moderating the nanoparticle dissolution, indicating limited bioavailability of the NPs upon transformation. The results improve the understanding of the fate and hazards of n-ZnO.
中文翻译:

纳米氧化锌在模拟汗液中的化学转化及其对抗菌功效的影响
纳米级氧化锌(n-ZnO)广泛用于个人护理产品和纺织品中,因此很可能会释放到人的汗液中。为了更好地评估n-ZnO的潜在人类健康风险,必须了解其在生理溶液(例如人的汗液)中的化学转化以及n-ZnO生物利用度的变化。在此,合成了两种类型的n-ZnO,ZnO纳米颗粒(ZnO-NPs)和基于纳米棒的ZnO纳米球(ZnO-NSs),并在3种类型的具有不同pH值和磷酸盐浓度的模拟汗液中进行了培养。通过选择性溶解Zn 3(PO 4)2来量化转化的n-ZnO中Zn 3(PO 4)2的含量。在0.35 M的氨溶液中溶解100%和5.5%的Zn 3(PO 4)2和ZnO。动力学分析表明,到24-48小时,Zn 3(PO 4)2的含量达到最大值,在pH 8.0时为15-21%,在pH 5.5或4.3时为45-70%。有趣的是,没有观察到Zn 3(PO 4)2形成的速率常数与n-ZnO的比表面积之间的相关性,这意味着从n-ZnO到Zn 3(PO 4)2的化学转化。模拟汗水中的碳可能不能简单地归因于溶解和沉淀。使用各种表征技术,我们证明了ZnO-Zn 3(PO 4)2核-壳结构的形成,该壳由pH 8.0的无定形Zn 3(PO 4)2和结晶的Zn 3(PO)组成4)2和Zn 3(PO 4)2 •4H 2pH为5.5或4.3的O。在pH 5.5和4.3的模拟汗液中磷酸盐诱导的n-ZnO转化通过减缓纳米颗粒的溶解而大大降低了n-ZnO的抗菌功效,表明转化后NP的生物利用度有限。结果提高了对n-ZnO的命运和危害的认识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号