Biomaterials ( IF 14.0 ) Pub Date : 2020-11-17 , DOI: 10.1016/j.biomaterials.2020.120537 Jingke Fu , Tao Li , Yangzi Yang , Liping Jiang , Wenhao Wang , Lingjie Fu , Yingchun Zhu , Yongqiang Hao
|
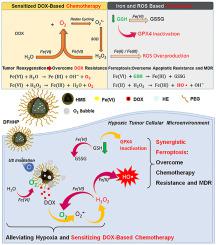
Hypoxia has been firmly correlated to the drug resistance of solid tumors. Alleviation of hypoxia by tumor reoxygenation is expected to sensitize the chemotherapy toward solid tumors. Alternatively, ferroptosis provides a therapeutic strategy to overcome apoptotic resistance and multidrug resistance of solid tumors, collaboratively strengthening the chemotherapy toward hypoxic tumors. Herein, an ultrasound (US)-activatable nanomedicine was developed for overcoming hypoxia-induced resistance to chemotherapy and efficiently inhibiting tumor growth by inducing sensitized apoptosis and collaborative ferroptosis of tumor cells. This nanomedicine was constructed by integrating ferrate and doxorubicin into biocompatible hollow mesoporous silica nanoplatforms, followed by assembling a solid-liquid phase-change material of n-heneicosane. The US-induced mild hyperthermia initiates the phase change of n-heneicosane, enabling US-activated co-release of ferrate and doxorubicin. Results reveal that the released ferrate effectively reacts with water as well as the over-expressed hydrogen peroxide and glutathione in tumor cells, achieving tumor-microenvironment-independent reoxygenation and glutathione-depletion in tumors. The reoxygenation down-regulates expressions of hypoxia-inducible factor 1α and multidrug resistance gene/transporter P-glycoprotein in tumor cells, sensitizing the apoptosis-based doxorubicin chemotherapy. More importantly, exogenous iron metabolism from the nanomedicine initiates intracellular Fenton reactions, leading to reactive oxygen species overproduction and iron-dependent ferroptotic death of tumor cells. Furthermore, the glutathione-depletion inactivates the glutathione peroxidase 4 (GPX4, a critical regulatory target in ferroptosis), inhibiting the reduction of lipid peroxides and reinforcing the ferroptotic cell death. The sensitized chemotherapy together with the iron-dependent ferroptosis of tumor cells play a synergistic role in boosting the growth suppression of hypoxic osteosarcoma in vivo. Additionally, the nanomedicine acts as a nanoprobe for in vivo photoacoustic imaging and glutathione tracking, showing great potential as theranostic agents for hypoxic solid tumors treatment.
中文翻译:

可激活的纳米药物,通过在实体瘤中诱导协同凋亡和肥大症,克服低氧诱导的对化疗的抗性并抑制肿瘤的生长
缺氧与实体瘤的耐药性密切相关。预期通过肿瘤复氧减轻缺氧将使化学疗法对实体瘤敏感。或者,肥大症提供了一种治疗策略,可以克服实体瘤的凋亡抗性和多药耐药性,从而协同增强针对缺氧肿瘤的化学疗法。在本文中,开发了超声(US)激活的纳米药物,用于克服缺氧诱导的对化学疗法的抵抗力,并通过诱导肿瘤细胞的敏化凋亡和协同肥大作用来有效抑制肿瘤生长。此纳米被积分的高铁酸盐和阿霉素成生物相容的中空介孔二氧化硅纳米平台,随后组装的固-液相变材料构成Ñ-二十二烷。美国引起的轻度高热引发n的相变-henesecosane,可实现美国激活的高铁酸盐和阿霉素的共释放。结果表明,释放的高铁酸盐与水以及过表达的过氧化氢和谷胱甘肽在肿瘤细胞中有效反应,从而在肿瘤中实现了与肿瘤微环境无关的复氧和谷胱甘肽耗竭。复氧作用下调肿瘤细胞中缺氧诱导因子1α和多药耐药基因/转运蛋白P糖蛋白的表达,从而使基于凋亡的阿霉素化疗变得敏感。更重要的是,来自纳米药物的外源铁代谢会引发细胞内Fenton反应,从而导致活性氧过度生成以及肿瘤细胞铁依赖的铁肥大性死亡。此外,谷胱甘肽耗竭会使谷胱甘肽过氧化物酶4(GPX4,铁通症的关键调控目标),抑制脂质过氧化物的减少并增强铁通性细胞的死亡。致敏化学疗法与肿瘤细胞铁依赖性肥大症一起,在促进缺氧性骨肉瘤生长抑制中起协同作用体内。此外,纳米药物还可以作为体内光声成像和谷胱甘肽追踪的纳米探针,显示出作为治疗低氧性实体瘤的治疗药物的巨大潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号