当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microwave-assisted periselective annulation of triarylphosphenes with aldehydes and ketones
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-11-10 , DOI: 10.1039/d0ob02011g Yun Luo 1 , Zhicheng Fu 1 , Xingyang Fu 1 , Changle Du 1 , Jiaxi Xu 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-11-10 , DOI: 10.1039/d0ob02011g Yun Luo 1 , Zhicheng Fu 1 , Xingyang Fu 1 , Changle Du 1 , Jiaxi Xu 1
Affiliation
|
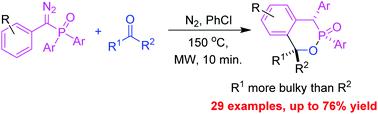
The reaction of diazo(aryl)methyl(diaryl)phosphine oxides with aldehydes and ketones generates benzo-δ-phosphinolactones in low to good yields with 1,1-diarylalk-1-enes as byproducts under microwave irradiation. Diazo(aryl)methyl(diaryl)phosphine oxides first undergo a Wolff rearrangement to form diaryl(aryl)phosphenes, which further react with aldehydes and ketones to afford benzo-δ-phosphinolactones and β-phosphinolactones. The latter are unstable under heating and fragment into the corresponding 1,1-diarylalk-1-enes and arylphosphine dioxides under reaction conditions. The arylphosphine dioxides become arylphosphonic acids during workup. The periselectivity in the annulation shows that the reaction of diaryl(aryl)phosphenes with most aldehydes and ketones favors phosphene phenyl participation in (4 + 2) annulation over (2 + 2) annulation.
中文翻译:

三芳基膦与醛和酮的微波辅助选择性环化
重氮(芳基)甲基(二芳基)氧化膦与醛和酮的反应生成苯并-δ-膦内酯,收率从低到高,1,1-二芳基烷-1-烯在微波照射下作为副产物。重氮(芳基)甲基(二芳基)氧化膦首先经历 Wolff 重排以形成二芳基(芳基)膦,其进一步与醛和酮反应生成苯并-δ-膦内酯和 β-膦内酯。后者在加热下不稳定并在反应条件下分裂成相应的1,1-二芳基烷-1-烯和芳基二氧化膦。芳基二氧化膦在后处理过程中变成芳基膦酸。环化中的选择性表明二芳基(芳基)膦与大多数醛和酮的反应有利于膦苯基参与(4 + 2)环化而不是(2 + 2)环化。
更新日期:2020-11-16
中文翻译:

三芳基膦与醛和酮的微波辅助选择性环化
重氮(芳基)甲基(二芳基)氧化膦与醛和酮的反应生成苯并-δ-膦内酯,收率从低到高,1,1-二芳基烷-1-烯在微波照射下作为副产物。重氮(芳基)甲基(二芳基)氧化膦首先经历 Wolff 重排以形成二芳基(芳基)膦,其进一步与醛和酮反应生成苯并-δ-膦内酯和 β-膦内酯。后者在加热下不稳定并在反应条件下分裂成相应的1,1-二芳基烷-1-烯和芳基二氧化膦。芳基二氧化膦在后处理过程中变成芳基膦酸。环化中的选择性表明二芳基(芳基)膦与大多数醛和酮的反应有利于膦苯基参与(4 + 2)环化而不是(2 + 2)环化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号