当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cyclization of interlocked fumaramides into β-lactams: experimental and computational mechanistic assessment of the key intercomponent proton transfer and the stereocontrolling active pocket
Chemical Science ( IF 8.4 ) Pub Date : 2020-11-11 , DOI: 10.1039/d0sc05757f Alberto Martinez-Cuezva 1 , Aurelia Pastor 1 , Marta Marin-Luna 1 , Carmen Diaz-Marin 1 , Delia Bautista 2 , Mateo Alajarin 1 , Jose Berna 1
Chemical Science ( IF 8.4 ) Pub Date : 2020-11-11 , DOI: 10.1039/d0sc05757f Alberto Martinez-Cuezva 1 , Aurelia Pastor 1 , Marta Marin-Luna 1 , Carmen Diaz-Marin 1 , Delia Bautista 2 , Mateo Alajarin 1 , Jose Berna 1
Affiliation
|
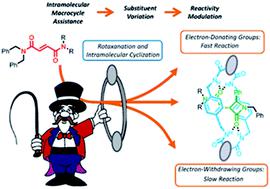
A detailed mechanistic study of the diastereoselective CsOH-promoted cyclization of interlocked fumaramides to give β-lactams is described. The mechanistic analysis comprises the experimental evaluation of the structure-reactivity relationship for a wide range of fumaramides [2]rotaxanes (Hammet-plots), KIE studies with deuterium-labelled interlocked fumaramides and computational analysis of two alternative mechanistic pathways for the cyclization process. The obtained results confirm that: (a) the rate-determining step is the deprotonation of the N-benzyl group of the thread by the amidate group of the macrocycle generated by the external base, (b) the polyamide macrocycle plays an important role not only as activating element but also as the stereodifferenciating factor responsible for the observed diastereoselection and (c) the higher flexibility of the adamantyl core speeds up the cyclization process in diadamantyl-derived rotaxanes.
中文翻译:

联锁富马酰胺环化成β-内酰胺:关键组分间质子转移和立体控制活性口袋的实验和计算机制评估
描述了非对映选择性 CsOH 促进的联锁富马酰胺环化以产生 β-内酰胺的详细机理研究。机理分析包括对多种富马酰胺 [2] 轮烷(Hammet-plots)的结构-反应性关系的实验评估、氘标记的互锁富马酰胺的 KIE 研究以及环化过程的两种替代机理途径的计算分析。获得的结果证实:(a)速率决定步骤是N的去质子化由外部碱生成的大环的酰胺基团的-苄基,(b)聚酰胺大环不仅作为活化元素,而且作为负责观察到的非对映选择的立体微分因子发挥重要作用和(c)金刚烷基核心的更高灵活性加速了金刚烷基衍生的轮烷中的环化过程。
更新日期:2020-11-16
中文翻译:

联锁富马酰胺环化成β-内酰胺:关键组分间质子转移和立体控制活性口袋的实验和计算机制评估
描述了非对映选择性 CsOH 促进的联锁富马酰胺环化以产生 β-内酰胺的详细机理研究。机理分析包括对多种富马酰胺 [2] 轮烷(Hammet-plots)的结构-反应性关系的实验评估、氘标记的互锁富马酰胺的 KIE 研究以及环化过程的两种替代机理途径的计算分析。获得的结果证实:(a)速率决定步骤是N的去质子化由外部碱生成的大环的酰胺基团的-苄基,(b)聚酰胺大环不仅作为活化元素,而且作为负责观察到的非对映选择的立体微分因子发挥重要作用和(c)金刚烷基核心的更高灵活性加速了金刚烷基衍生的轮烷中的环化过程。


























 京公网安备 11010802027423号
京公网安备 11010802027423号