当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Acyclic nitronate olefin cycloaddition (ANOC): regio- and stereospecific synthesis of isoxazolines
Chemical Science ( IF 8.4 ) Pub Date : 2020-11-06 , DOI: 10.1039/d0sc05607c Liang Ma 1 , Luyao Kou 1 , Feng Jin 1 , Xionglve Cheng 1 , Suyan Tao 1 , Gangzhong Jiang 1 , Xiaoguang Bao 1 , Xiaobing Wan 1
Chemical Science ( IF 8.4 ) Pub Date : 2020-11-06 , DOI: 10.1039/d0sc05607c Liang Ma 1 , Luyao Kou 1 , Feng Jin 1 , Xionglve Cheng 1 , Suyan Tao 1 , Gangzhong Jiang 1 , Xiaoguang Bao 1 , Xiaobing Wan 1
Affiliation
|
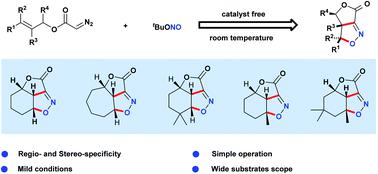
We report the first demonstrations of intra- and intermolecular acyclic nitronate olefin cycloaddition (ANOC) reactions that enable the highly efficient syntheses of isoxazolines bearing various functional groups. This general approach to accessing γ-lactone fused isoxazolines was hitherto unprecedented. The room temperature transformations reported herein exhibit wide substrate scopes, as evidenced by more than 70 examples, including the generation of five tricyclic isoxazolines. The robustness of this methodology was confirmed by a series of trials that afforded highly functionalized isoxazolines. Both experimental results and density functional theory calculations indicate that these transformations proceed via the in situ formation of acyclic nitronates together with concerted [3+2] cycloaddition and tert-butyloxy group elimination processes to give regio- and stereospecificity.
中文翻译:

无环亚硝酸盐烯烃环加成(ANOC):异恶唑啉的区域和立体定向合成
我们报告的第一个示范的分子内和分子间的无环亚硝酸盐烯烃环加成(ANOC)反应,使带有各种官能团的异恶唑啉的高效合成成为可能。迄今为止,这种访问γ-内酯稠合的异恶唑啉的通用方法是前所未有的。如70多个实例所示,本文报道的室温转化表现出较宽的底物范围,包括生成五种三环异恶唑啉。通过提供高度功能化的异恶唑啉的一系列试验证实了该方法的鲁棒性。两个实验结果和密度泛函理论计算表明,这些变换继续经由所述原位形成无环的硝酸盐以及协同的[3 + 2]环加成反应和叔丁氧基消除过程,从而获得区域和立体特异性。
更新日期:2020-11-16
中文翻译:

无环亚硝酸盐烯烃环加成(ANOC):异恶唑啉的区域和立体定向合成
我们报告的第一个示范的分子内和分子间的无环亚硝酸盐烯烃环加成(ANOC)反应,使带有各种官能团的异恶唑啉的高效合成成为可能。迄今为止,这种访问γ-内酯稠合的异恶唑啉的通用方法是前所未有的。如70多个实例所示,本文报道的室温转化表现出较宽的底物范围,包括生成五种三环异恶唑啉。通过提供高度功能化的异恶唑啉的一系列试验证实了该方法的鲁棒性。两个实验结果和密度泛函理论计算表明,这些变换继续经由所述原位形成无环的硝酸盐以及协同的[3 + 2]环加成反应和叔丁氧基消除过程,从而获得区域和立体特异性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号