当前位置:
X-MOL 学术
›
Adv. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical Oxygen Reduction to Hydrogen Peroxide via a Two‐Electron Transfer Pathway on Carbon‐Based Single‐Atom Catalysts
Advanced Materials Interfaces ( IF 5.4 ) Pub Date : 2020-11-16 , DOI: 10.1002/admi.202001360 Kai Sun 1 , Wenwen Xu 2 , Xiao Lin 3 , Shubo Tian 1 , Wen‐Feng Lin 4 , Daojin Zhou 1 , Xiaoming Sun 1
Advanced Materials Interfaces ( IF 5.4 ) Pub Date : 2020-11-16 , DOI: 10.1002/admi.202001360 Kai Sun 1 , Wenwen Xu 2 , Xiao Lin 3 , Shubo Tian 1 , Wen‐Feng Lin 4 , Daojin Zhou 1 , Xiaoming Sun 1
Affiliation
|
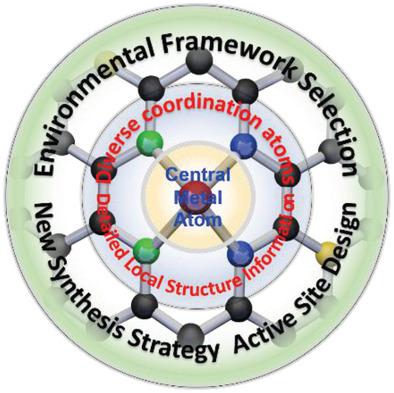
Electrochemical reduction of oxygen is considered as a new strategy to achieve decentralized preparation of hydrogen peroxide (H2O2) in a green manner. As a promising new type of catalytic material, carbon‐based single‐atom catalysts can achieve wide‐range adjustments of the electronic structure of the active metal centers while also maximize the utilization of metal atoms, toward electrochemical production of H2O2 from the selective two‐electron transfer oxygen reduction reaction (ORR). Herein, starting from the reviewing of characterizing methods and reaction mechanisms of ORR via two‐electron and four‐electron transfer pathways, the vital role of binding strength between OOH intermediate and active sites in determining the activity and selectivity towards H2O2 production is revealed and illustrated. Currently reported carbon‐based single‐atom catalysts for H2O2 production are systematically summarized and critically reviewed. Moreover, with the underpinning chemistry to improve the overall efficiency, three aspects concerning the central metal atoms, coordinated atoms, and environmental atoms are comprehensively analyzed. Based on the understanding of the most current progresses, some predictions for future H2O2 production via electrochemical routes are offered, which include catalyst designs at atomic levels, new synthesis strategies and characterization techniques, as well as interfacial superwetting interaction engineering at electrode and device levels.
中文翻译:

碳基单原子催化剂上通过双电子转移途径将电化学氧还原为过氧化氢
电化学还原氧气被认为是一种以绿色方式分散制备过氧化氢(H 2 O 2)的新策略。碳基单原子催化剂作为一种有前途的新型催化材料,可以实现活性金属中心电子结构的宽范围调节,同时还可以最大程度地利用金属原子,从而实现H 2 O 2的电化学生产。来自选择性二电子转移氧还原反应(ORR)。在此,从回顾通过两电子和四电子转移途径进行的ORR的表征方法和反应机理开始,OOH中间位点和活性位点之间的结合强度在确定H 2 O 2产生的活性和选择性方面的至关重要的作用是揭示和说明。目前报道的H 2 O 2碳基单原子催化剂对生产进行了系统总结和严格审查。此外,通过提高整体效率的化学基础,对中心金属原子,配位原子和环境原子三个方面进行了综合分析。在了解最新进展的基础上,提供了对未来通过电化学途径生产H 2 O 2的一些预测,包括在原子水平上的催化剂设计,新的合成策略和表征技术,以及电极和电极之间的界面超湿相互作用工程。设备级别。
更新日期:2020-11-16
中文翻译:

碳基单原子催化剂上通过双电子转移途径将电化学氧还原为过氧化氢
电化学还原氧气被认为是一种以绿色方式分散制备过氧化氢(H 2 O 2)的新策略。碳基单原子催化剂作为一种有前途的新型催化材料,可以实现活性金属中心电子结构的宽范围调节,同时还可以最大程度地利用金属原子,从而实现H 2 O 2的电化学生产。来自选择性二电子转移氧还原反应(ORR)。在此,从回顾通过两电子和四电子转移途径进行的ORR的表征方法和反应机理开始,OOH中间位点和活性位点之间的结合强度在确定H 2 O 2产生的活性和选择性方面的至关重要的作用是揭示和说明。目前报道的H 2 O 2碳基单原子催化剂对生产进行了系统总结和严格审查。此外,通过提高整体效率的化学基础,对中心金属原子,配位原子和环境原子三个方面进行了综合分析。在了解最新进展的基础上,提供了对未来通过电化学途径生产H 2 O 2的一些预测,包括在原子水平上的催化剂设计,新的合成策略和表征技术,以及电极和电极之间的界面超湿相互作用工程。设备级别。


























 京公网安备 11010802027423号
京公网安备 11010802027423号