Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2020-11-16 , DOI: 10.1016/j.bmc.2020.115874 Sandeep Sundriyal 1 , Venkateswararao Eeda 1 , Pallavi Lagisetty 1 , Vibhudutta Awasthi 1
|
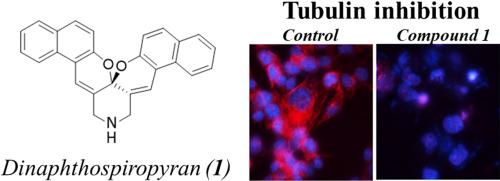
Spiropyrans have been investigated for their thermo- and photochromic characteristics, but their biotherapeutic properties have not been addressed. We report anti-proliferative properties of a novel dinaphthospiropyran analogue (1). The compound 1 was synthesized by a simple and expedient method using a one-pot acid-catalyzed aldol condensation of 2-hydroxy-1-naphthaldehyde with 4-piperidone followed by an acetalization reaction. Compound 1 was submitted to anticancer drug screen in the National Cancer Institute’s panel of 60 human tumor cell lines. The average concentration of 1 to inhibit 50% cell growth was 5.4 ± 0.23 µM. All cell lines responded at almost the same concentration, suggesting that the action of 1 is not selective for cancer of origin. COMPARE analysis of dose-response data revealed interaction with tubulin as the possible mechanism of action of 1. At molecular level, 1 induced tubulin reorganization in colon cancer HCT-116 cells. Under cell-free conditions, the efficacy of 1 to inhibit tubulin polymerization was comparable to that of paclitaxel and vinblastine. Molecular docking showed that compound 1 binds to the colchicine-binding site of tubulin. We conclude that dinaphthospiropyrans present a novel scaffold for the development of tubulin inhibitors.
中文翻译:

基于二萘螺吡喃支架的新型秋水仙碱结合化合物的微管蛋白抑制活性
已经研究了螺吡喃的热致变色和光致变色特性,但尚未解决它们的生物治疗特性。我们报告了一种新型二萘螺吡喃类似物的抗增殖特性 ( 1)。使用一锅酸催化的 2-羟基-1-萘醛与 4-哌啶酮的醛醇缩合,然后进行缩醛化反应,通过简单而方便的方法合成了化合物1。化合物1被提交到美国国家癌症研究所的 60 个人类肿瘤细胞系面板中进行抗癌药物筛选。1抑制 50% 细胞生长的平均浓度为 5.4 ± 0.23 µM。所有细胞系的反应浓度几乎相同,表明1对原发癌没有选择性。剂量反应数据的比较分析揭示了与微管蛋白的相互作用是1的可能作用机制。在分子水平上,1诱导结肠癌 HCT-116 细胞中的微管蛋白重组。在无细胞条件下,1抑制微管蛋白聚合的功效与紫杉醇和长春碱的功效相当。分子对接表明,化合物1与微管蛋白的秋水仙碱结合位点结合。我们得出结论,dinaphthospiropyrans 为微管蛋白抑制剂的开发提供了一种新的支架。


























 京公网安备 11010802027423号
京公网安备 11010802027423号