当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational and Experimental Evaluation of Peroxide Oxidants for Amine–Peroxide Redox Polymerization
Macromolecules ( IF 5.5 ) Pub Date : 2020-11-13 , DOI: 10.1021/acs.macromol.0c02069 Charles B Musgrave 1 , Kangmin Kim 2 , Nicholas R Singstock 3 , Austyn M Salazar 4 , Jeffrey W Stansbury 4 , Charles B Musgrave 5
Macromolecules ( IF 5.5 ) Pub Date : 2020-11-13 , DOI: 10.1021/acs.macromol.0c02069 Charles B Musgrave 1 , Kangmin Kim 2 , Nicholas R Singstock 3 , Austyn M Salazar 4 , Jeffrey W Stansbury 4 , Charles B Musgrave 5
Affiliation
|
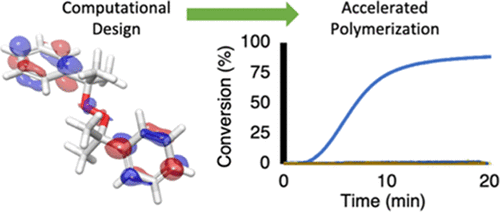
Amine–peroxide redox polymerization (APRP) is the prevalent method for producing radical-based polymers in the many industrial and medical applications where light or heat activation is impractical. We recently developed a detailed description of the APRP initiation process through a combined computational and experimental effort to show that APRP proceeds through SN2 attack by the amine on the peroxide, followed by the rate-determining homolysis of the resulting intermediate. Using this new mechanistic understanding, a variety of peroxides were computationally predicted to initiate APRP with fast kinetics. In particular, the rate of APRP initiation can be improved by radical and anion stabilization through increased π-electron conjugation or by increasing the electrophilicity of the peroxy bond through the addition of electron-withdrawing groups. On the other hand, the addition of electron-donating groups lowered the initiation rate. These design principles enabled the computational prediction of several new peroxides that exhibited improved initiation rates over the commonly used benzoyl peroxide. For example, the addition of nitro groups (NO2) to the para positions of benzoyl peroxide resulted in a theoretical radical generation rate of 1.9 × 10–9 s–1, which is ∼150 times faster than the 1.3 × 10–11 s–1 radical generation rate observed with unsubstituted benzoyl peroxide. These accelerated kinetics enabled the development of a redox-based direct-writing process that exploited the extremely rapid reactivity of an optimized redox pair with a custom inkjet printer, capable of printing custom shapes from polymerizing resins without heat or light. Furthermore, the application of more rapid APRP kinetics could enable the acceleration of existing industrial processes, make new industrial manufacturing methods possible, and improve APRP compatibility with biomedical applications through reduced initiator concentrations that still produce rapid polymerization rates.
中文翻译:

用于胺-过氧化物氧化还原聚合的过氧化物氧化剂的计算和实验评价
胺-过氧化物氧化还原聚合 (APRP) 是在许多工业和医疗应用中生产自由基聚合物的普遍方法,在这些应用中光或热活化是不切实际的。我们最近通过结合计算和实验努力开发了 APRP 启动过程的详细描述,以表明 APRP 通过 S N进行2 胺对过氧化物的攻击,然后是所得中间体的决定速率的均裂。使用这种新的机制理解,计算预测各种过氧化物会以快速动力学启动 APRP。特别是,通过增加 π 电子共轭或通过添加吸电子基团增加过氧键的亲电性,可以通过自由基和阴离子稳定来提高 APRP 引发的速率。另一方面,给电子基团的加入降低了引发速率。这些设计原则能够计算预测几种新的过氧化物,这些过氧化物比常用的过氧化苯甲酰具有更高的引发率。例如,添加硝基(NO 2) 对过氧化苯甲酰的对位产生 1.9 × 10 –9 s –1的理论自由基生成速率,比 1.3 × 10 –11 s –1快 ∼150 倍用未取代的过氧化苯甲酰观察到的自由基生成速率。这些加速动力学使基于氧化还原的直接写入工艺得以开发,该工艺利用优化的氧化还原对与定制喷墨打印机的极快反应性,能够在没有热或光的情况下从聚合树脂打印定制形状。此外,更快速的 APRP 动力学的应用可以加速现有的工业过程,使新的工业制造方法成为可能,并通过降低引发剂浓度而提高 APRP 与生物医学应用的兼容性,从而仍然产生快速聚合速率。
更新日期:2020-11-25
中文翻译:

用于胺-过氧化物氧化还原聚合的过氧化物氧化剂的计算和实验评价
胺-过氧化物氧化还原聚合 (APRP) 是在许多工业和医疗应用中生产自由基聚合物的普遍方法,在这些应用中光或热活化是不切实际的。我们最近通过结合计算和实验努力开发了 APRP 启动过程的详细描述,以表明 APRP 通过 S N进行2 胺对过氧化物的攻击,然后是所得中间体的决定速率的均裂。使用这种新的机制理解,计算预测各种过氧化物会以快速动力学启动 APRP。特别是,通过增加 π 电子共轭或通过添加吸电子基团增加过氧键的亲电性,可以通过自由基和阴离子稳定来提高 APRP 引发的速率。另一方面,给电子基团的加入降低了引发速率。这些设计原则能够计算预测几种新的过氧化物,这些过氧化物比常用的过氧化苯甲酰具有更高的引发率。例如,添加硝基(NO 2) 对过氧化苯甲酰的对位产生 1.9 × 10 –9 s –1的理论自由基生成速率,比 1.3 × 10 –11 s –1快 ∼150 倍用未取代的过氧化苯甲酰观察到的自由基生成速率。这些加速动力学使基于氧化还原的直接写入工艺得以开发,该工艺利用优化的氧化还原对与定制喷墨打印机的极快反应性,能够在没有热或光的情况下从聚合树脂打印定制形状。此外,更快速的 APRP 动力学的应用可以加速现有的工业过程,使新的工业制造方法成为可能,并通过降低引发剂浓度而提高 APRP 与生物医学应用的兼容性,从而仍然产生快速聚合速率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号