当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct and Precise Measurement of Bevacizumab Levels in Human Plasma Based on Controlled Methionine Oxidation and Multiple Reaction Monitoring
ACS Pharmacology & Translational Science Pub Date : 2020-11-13 , DOI: 10.1021/acsptsci.0c00134 Vanessa P Gaspar 1 , Sahar Ibrahim 1 , Constance A Sobsey 1 , Vincent R Richard 2 , Alan Spatz 3 , René P Zahedi 2, 4 , Christoph H Borchers 1, 4
ACS Pharmacology & Translational Science Pub Date : 2020-11-13 , DOI: 10.1021/acsptsci.0c00134 Vanessa P Gaspar 1 , Sahar Ibrahim 1 , Constance A Sobsey 1 , Vincent R Richard 2 , Alan Spatz 3 , René P Zahedi 2, 4 , Christoph H Borchers 1, 4
Affiliation
|
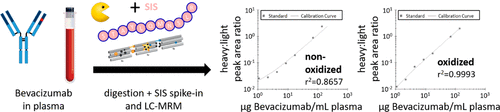
Bevacizumab is a monoclonal antibody which targets vascular endothelial growth factor A (VEGF-A) and is used to treat various cancers and recently COVID-19. The dosage recommendations for bevacizumab are determined on the basis of body weight, and the drug is administered after defined time intervals, when it is presumed to still be above its minimum effective serum concentration. Interindividual and disease-stage-related variations in bevacizumab catabolism, however, can affect the proper dosing of patients, resulting in plasma concentrations which may not be within the optimal therapeutic window for the drug. Therapeutic drug monitoring (TDM) enables the assessment of patients’ serum concentrations and allows personalized dosing which has the potential to improve efficacy and reduce side effects. While TMD is often performed using ligand-based assays, mass spectrometry (MS)-based TDM offers improved specificity. Here, we present a robust multiple reaction monitoring (MRM)-MS-based TDM method for the precise quantification of bevacizumab plasma concentrations, based on the controlled oxidation of the methionine-containing peptide, STAYLQMNSLR. The assay shows good linearity (r2 = 0.9951), robustness, and precision (CVs < 20%) for the quantification of bevacizumab, with a lower limit of quantification (S/N > 10) of 1.8 μg/mL of plasma, without the need for enrichment and requiring less than 1 μL of plasma and less than 6 h from sampling to result.
中文翻译:

基于受控蛋氨酸氧化和多反应监测直接和精确测量人血浆中的贝伐单抗水平
贝伐珠单抗是一种靶向血管内皮生长因子 A (VEGF-A) 的单克隆抗体,用于治疗各种癌症和最近的 COVID-19。贝伐珠单抗的推荐剂量是根据体重确定的,在规定的时间间隔后给药,当它被假定仍高于其最低有效血清浓度时。然而,贝伐单抗分解代谢的个体间和疾病阶段相关的差异会影响患者的正确给药,导致血浆浓度可能不在药物的最佳治疗窗口内。治疗药物监测 (TDM) 能够评估患者的血清浓度,并允许个性化给药,这有可能提高疗效并减少副作用。虽然 TMD 通常使用基于配体的检测进行,但基于质谱 (MS) 的 TDM 提供了更高的特异性。在这里,我们提出了一种强大的基于多反应监测 (MRM)-MS 的 TDM 方法,用于精确量化贝伐单抗血浆浓度,基于含蛋氨酸的肽 STAYLQMNSLR 的受控氧化。该测定显示出良好的线性(r 2 = 0.9951)、稳健性和精确度 (CVs < 20%) 用于贝伐单抗的定量,定量下限 ( S / N > 10) 为 1.8 μg/mL 血浆,无需富集且要求血浆少于 1 μL,从采样到结果少于 6 小时。
更新日期:2020-12-12
中文翻译:

基于受控蛋氨酸氧化和多反应监测直接和精确测量人血浆中的贝伐单抗水平
贝伐珠单抗是一种靶向血管内皮生长因子 A (VEGF-A) 的单克隆抗体,用于治疗各种癌症和最近的 COVID-19。贝伐珠单抗的推荐剂量是根据体重确定的,在规定的时间间隔后给药,当它被假定仍高于其最低有效血清浓度时。然而,贝伐单抗分解代谢的个体间和疾病阶段相关的差异会影响患者的正确给药,导致血浆浓度可能不在药物的最佳治疗窗口内。治疗药物监测 (TDM) 能够评估患者的血清浓度,并允许个性化给药,这有可能提高疗效并减少副作用。虽然 TMD 通常使用基于配体的检测进行,但基于质谱 (MS) 的 TDM 提供了更高的特异性。在这里,我们提出了一种强大的基于多反应监测 (MRM)-MS 的 TDM 方法,用于精确量化贝伐单抗血浆浓度,基于含蛋氨酸的肽 STAYLQMNSLR 的受控氧化。该测定显示出良好的线性(r 2 = 0.9951)、稳健性和精确度 (CVs < 20%) 用于贝伐单抗的定量,定量下限 ( S / N > 10) 为 1.8 μg/mL 血浆,无需富集且要求血浆少于 1 μL,从采样到结果少于 6 小时。



























 京公网安备 11010802027423号
京公网安备 11010802027423号