当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A base-catalyzed domino reaction between isoindigos and α-alkylidene succinimides—convenient preparation of highly steric bispirooxindoles
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-11-04 , DOI: 10.1039/d0ob01858a Chen-Yi Li 1 , Min Xiang 1 , Xiang-Jia Song 1 , Ying Zou 1 , Zhi-Cheng Huang 1 , Xia Li 1 , Fang Tian 2 , Li-Xin Wang 2
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-11-04 , DOI: 10.1039/d0ob01858a Chen-Yi Li 1 , Min Xiang 1 , Xiang-Jia Song 1 , Ying Zou 1 , Zhi-Cheng Huang 1 , Xia Li 1 , Fang Tian 2 , Li-Xin Wang 2
Affiliation
|
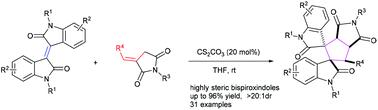
A new base promoted Michael–Michael domino cycloaddition between isoindigos and α-alkylidene succinimides has been developed for highly efficient and one-step convenient preparation of highly steric bispiroxindoles with two adjacent quaternary carbon centers and four consecutive cycles in excellent yields (up to 96%) and diastereoselectivities (up to >20 : 1) under mild conditions within a few minutes. A series of bisprooxindoles were obtained and the synthetic potential of the protocol was evaluated in a scale-up preparation.
中文翻译:

异靛蓝与α-亚烷基琥珀酰亚胺之间的碱催化多米诺反应——高空间位阻双螺吲哚的简便制备
已经开发了一种促进异靛蓝和α-亚烷基琥珀酰亚胺之间的迈克尔-迈克尔多米诺环加成的新碱基,用于高效和一步方便地制备具有两个相邻季碳中心和四个连续循环的高位阻双螺吲哚,产率高达 96% ) 和非对映选择性(高达 >20 : 1)在温和条件下几分钟内。获得了一系列双丙氧吲哚,并在放大制备中评估了该方案的合成潜力。
更新日期:2020-11-13
中文翻译:

异靛蓝与α-亚烷基琥珀酰亚胺之间的碱催化多米诺反应——高空间位阻双螺吲哚的简便制备
已经开发了一种促进异靛蓝和α-亚烷基琥珀酰亚胺之间的迈克尔-迈克尔多米诺环加成的新碱基,用于高效和一步方便地制备具有两个相邻季碳中心和四个连续循环的高位阻双螺吲哚,产率高达 96% ) 和非对映选择性(高达 >20 : 1)在温和条件下几分钟内。获得了一系列双丙氧吲哚,并在放大制备中评估了该方案的合成潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号