当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Emergence of Barrel Motif in Amyloid-β Trimer: A Computational Study
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.jpcb.0c05508 Hoang Linh Nguyen 1, 2, 3 , Huynh Quang Linh 2, 3 , Paolo Matteini 4 , Giovanni La Penna 5 , Mai Suan Li
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.jpcb.0c05508 Hoang Linh Nguyen 1, 2, 3 , Huynh Quang Linh 2, 3 , Paolo Matteini 4 , Giovanni La Penna 5 , Mai Suan Li
Affiliation
|
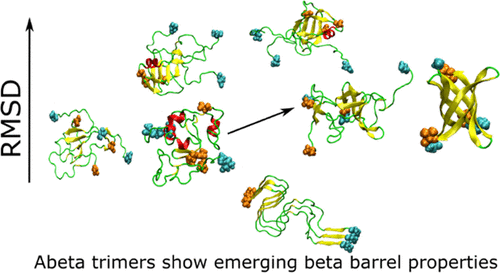
Amyloid-β (Aβ) peptides form assemblies that are pathological hallmarks of Alzheimer’s disease. Aβ oligomers are soluble, mobile, and toxic forms of the peptide that act in the extracellular space before assembling into protofibrils and fibrils. Therefore, oligomers play an important role in the mechanism of Alzheimer’s disease. Since it is difficult to determine by experiment the atomic structures of oligomers, which accumulate fast and are polymorphic, computer simulation is a useful tool to investigate elusive oligomers’ structures. In this work, we report extended all-atom molecular dynamics simulations, both canonical and replica exchange, of Aβ(1–42) trimer starting from two different initial conformations: (i) the pose produced by the best docking of a monomer aside of a dimer (simulation 1), representing oligomers freshly formed by assembling monomers, and (ii) a configuration extracted from an experimental mature fibril structure (simulation 2), representing settled oligomers in equilibrium with extended fibrils. We showed that in simulation 1, regions with small β-barrels are populated, indicating the chance of spontaneous formation of domains resembling channel-like structures. These structural domains are alternative to those more representative of mature fibrils (simulation 2), the latter showing a stable bundle of C-termini that is not sampled in simulation 1. Moreover, trimer of Aβ(1–42) can form internal pores that are large enough to be accessed by water molecules and Ca2+ ions.
中文翻译:

淀粉样β三聚体中桶基元的出现:计算研究。
淀粉样蛋白-β(Aβ)肽形成的集合体是阿尔茨海默氏病的病理学标志。Aβ寡聚物是肽的可溶,可移动和有毒形式,可在组装成原纤维和原纤维之前作用于细胞外空间。因此,低聚物在阿尔茨海默氏病的机制中起着重要作用。由于很难通过实验确定低聚物的原子结构,这些原子快速积累并且是多态的,因此计算机仿真是研究难以捉摸的低聚物结构的有用工具。在这项工作中,我们报告了Aβ(1-42)三聚体从两个不同的初始构象开始的扩展的全原子分子动力学模拟,包括规范的和复制的交换:(i)由单体的最佳对接形成的姿势二聚体(模拟1),代表通过组装单体新鲜形成的低聚物,和(ii)从实验成熟的原纤维结构中提取的构型(模拟2),代表与延伸的原纤维平衡的沉降的低聚物。我们显示,在模拟1中,填充了具有小β桶的区域,这表明类似通道样结构的域自发形成的机会。这些结构域可以替代那些更能代表成熟原纤维的结构域(模拟2),后者显示稳定的C末端束,在模拟1中未采样。此外,Aβ(1-42)的三聚体可形成足够大的内部孔,水分子和Ca 2+离子可进入该内部孔。
更新日期:2020-11-25
中文翻译:

淀粉样β三聚体中桶基元的出现:计算研究。
淀粉样蛋白-β(Aβ)肽形成的集合体是阿尔茨海默氏病的病理学标志。Aβ寡聚物是肽的可溶,可移动和有毒形式,可在组装成原纤维和原纤维之前作用于细胞外空间。因此,低聚物在阿尔茨海默氏病的机制中起着重要作用。由于很难通过实验确定低聚物的原子结构,这些原子快速积累并且是多态的,因此计算机仿真是研究难以捉摸的低聚物结构的有用工具。在这项工作中,我们报告了Aβ(1-42)三聚体从两个不同的初始构象开始的扩展的全原子分子动力学模拟,包括规范的和复制的交换:(i)由单体的最佳对接形成的姿势二聚体(模拟1),代表通过组装单体新鲜形成的低聚物,和(ii)从实验成熟的原纤维结构中提取的构型(模拟2),代表与延伸的原纤维平衡的沉降的低聚物。我们显示,在模拟1中,填充了具有小β桶的区域,这表明类似通道样结构的域自发形成的机会。这些结构域可以替代那些更能代表成熟原纤维的结构域(模拟2),后者显示稳定的C末端束,在模拟1中未采样。此外,Aβ(1-42)的三聚体可形成足够大的内部孔,水分子和Ca 2+离子可进入该内部孔。


























 京公网安备 11010802027423号
京公网安备 11010802027423号