当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational NMR Study of Ion Pairing of 1-Decyl-3-methyl-imidazolium Chloride in Molecular Solvents
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.jpcb.0c07450 Dovilė Lengvinaitė 1 , Vytautas Klimavičius 1, 2 , Vytautas Balevicius 1 , Kęstutis Aidas 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.jpcb.0c07450 Dovilė Lengvinaitė 1 , Vytautas Klimavičius 1, 2 , Vytautas Balevicius 1 , Kęstutis Aidas 1
Affiliation
|
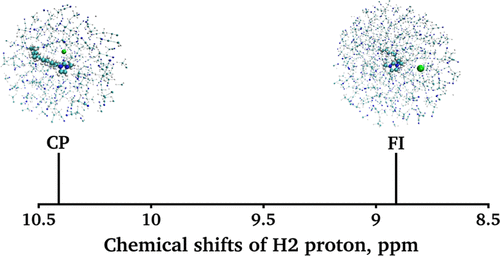
The 1H NMR spectra of 10–5 mole fraction solutions of 1-decyl-3-methyl-imidazolium chloride ionic liquid in water, acetonitrile, and dichloromethane have been measured. The chemical shift of the proton at position 2 in the imidazolium ring of 1-decyl-3-methyl-imidazolium (H2) is rather different for all three samples, reflecting the shifting equilibrium between the contact pairs and free fully solvated ions. Classical molecular dynamics simulations of the 1-decyl-3-methyl-imidazolium chloride contact ion pair as well as of free ions in water, acetonitrile, and dichloromethane have been conducted, and the quantum mechanics/molecular mechanics methods have been applied to predict NMR chemical shifts for the H2 proton. The chemical shift of the H2 proton was found to be primarily modulated by hydrogen bonding with the chloride anion, while the influence of the solvents—though differing in polarity and capabilities for hydrogen bonding—is less important. By comparing experimental and computational results, we deduce that complete disruption of the ionic liquid into free ions takes place in an aqueous solution. Around 23% of contact ion pairs were found to persist in acetonitrile. Ion-pair breaking into free ions was predicted not to occur in dichloromethane.
中文翻译:

分子溶剂中1-癸-3-甲基-咪唑鎓氯离子配对的计算核磁共振研究
的1 1 H NMR谱的10 -5测量了1-癸基-3-甲基-咪唑鎓氯化物离子液体在水,乙腈和二氯甲烷中的摩尔分数溶液。对于所有三个样品,在1-癸基-3-甲基-咪唑鎓(H2)的咪唑鎓环的位置2处的质子化学位移都非常不同,这反映了接触对与自由的完全溶剂化离子之间的位移平衡。对1-癸基-3-甲基-咪唑鎓氯化物接触离子对以及水,乙腈和二氯甲烷中的游离离子进行了经典的分子动力学模拟,并将量子力学/分子力学方法用于预测NMR H2质子的化学位移 发现氢质子的化学位移主要受与氯离子的氢键作用所调节,尽管极性和氢键结合能力不同,溶剂的影响并不重要。通过比较实验和计算结果,我们推断出离子液体完全分解为游离离子发生在水溶液中。发现约23%的接触离子对保留在乙腈中。预计在二氯甲烷中不会发生离子对分裂成自由离子的情况。
更新日期:2020-11-25
中文翻译:

分子溶剂中1-癸-3-甲基-咪唑鎓氯离子配对的计算核磁共振研究
的1 1 H NMR谱的10 -5测量了1-癸基-3-甲基-咪唑鎓氯化物离子液体在水,乙腈和二氯甲烷中的摩尔分数溶液。对于所有三个样品,在1-癸基-3-甲基-咪唑鎓(H2)的咪唑鎓环的位置2处的质子化学位移都非常不同,这反映了接触对与自由的完全溶剂化离子之间的位移平衡。对1-癸基-3-甲基-咪唑鎓氯化物接触离子对以及水,乙腈和二氯甲烷中的游离离子进行了经典的分子动力学模拟,并将量子力学/分子力学方法用于预测NMR H2质子的化学位移 发现氢质子的化学位移主要受与氯离子的氢键作用所调节,尽管极性和氢键结合能力不同,溶剂的影响并不重要。通过比较实验和计算结果,我们推断出离子液体完全分解为游离离子发生在水溶液中。发现约23%的接触离子对保留在乙腈中。预计在二氯甲烷中不会发生离子对分裂成自由离子的情况。



























 京公网安备 11010802027423号
京公网安备 11010802027423号