当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fabrication of Extremely Concentrated Silver Hydrosols without Additional Stabilizers
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-11-12 , DOI: 10.1021/acssuschemeng.0c06006 Sergey A. Vorobyev 1 , Maxim N. Likhatski 1 , Alexander S. Romanchenko 1 , Olga Y. Fetisova 1 , Alexander S. Kazachenko 1 , Mikhail N. Volochaev 2, 3 , Yuri L. Mikhlin 1
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-11-12 , DOI: 10.1021/acssuschemeng.0c06006 Sergey A. Vorobyev 1 , Maxim N. Likhatski 1 , Alexander S. Romanchenko 1 , Olga Y. Fetisova 1 , Alexander S. Kazachenko 1 , Mikhail N. Volochaev 2, 3 , Yuri L. Mikhlin 1
Affiliation
|
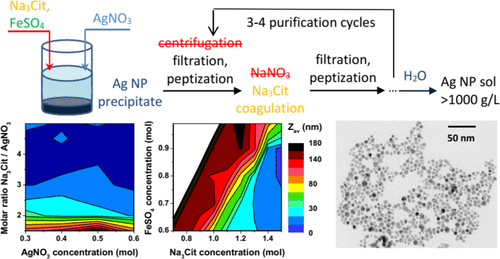
Applications of silver nanoparticles (Ag NPs) in modern technologies require environmentally friendly methods of large-scale production of the nanoparticles with controlled morphology and surface state in the form of high-concentration metal sols with minimal quantities of organic stabilizers. Herein, we report a procedure based on reduction of aqueous silver nitrate with ferrous sulfate in the presence of citrate ions. We studied the effect of various factors on the chemical reaction by applying transmission electron microscopy, ultraviolet–visible absorption spectroscopy, and dynamic light scattering (DLS) and proposed protocols with reduced quantities of the reagents allowing preparation of uniform spherical Ag NPs of 5 to 15 nm in diameter. A DLS study of sols after dilution was employed to estimate the tendency of colloidal particles to interact in order to optimize post-synthetic purification and concentration procedures. Particularly, filtration instead of centrifugation and electrolytic coagulation with trisodium citrate in place of sodium nitrate were utilized to produce extremely concentrated, more than 1000 g/L Ag, and stable silver hydrosols with no additional stabilizers. The chemical, X-ray photoelectron spectroscopy, and thermogravimetric analyses demonstrated that the Ag NPs contained citrate-derived capping ligands, and low amounts of Fe are appropriated for chemical and low-temperature sintering, surface functionalization, nanofluidics, and other applications.
中文翻译:

无需额外稳定剂即可制备高浓度银溶胶
银纳米颗粒(Ag NPs)在现代技术中的应用要求采用环境友好的方法,以高浓度金属溶胶和少量有机稳定剂的形式大规模生产具有受控形态和表面状态的纳米颗粒。本文中,我们报道了一种基于在柠檬酸根离子存在下用硫酸亚铁还原硝酸银水溶液的方法。我们通过应用透射电子显微镜,紫外-可见吸收光谱和动态光散射(DLS)研究了各种因素对化学反应的影响,并提出了减少试剂量的方案,从而可以制备5到15的均匀球形Ag NP直径nm。稀释后对溶胶进行DLS研究,以评估胶体颗粒相互作用的趋势,以优化合成后的纯化和浓缩程序。特别地,过滤代替离心和用柠檬酸三钠代替硝酸钠的电解凝结被用于生产极浓缩的,大于1000g / L的Ag和稳定的银水溶胶,而没有额外的稳定剂。化学,X射线光电子能谱和热重分析表明,Ag NP包含柠檬酸盐衍生的封端配体,少量的Fe适用于化学和低温烧结,表面功能化,纳米流体和其他应用。过滤代替离心分离,并用柠檬酸三钠代替硝酸钠进行电解凝结,可制得浓度极高,超过1000 g / L的Ag和稳定的银水溶胶,而无需额外的稳定剂。化学,X射线光电子能谱和热重分析表明,Ag NP包含柠檬酸盐衍生的封端配体,少量的Fe适用于化学和低温烧结,表面功能化,纳米流体和其他应用。过滤代替离心分离,并用柠檬酸三钠代替硝酸钠进行电解凝结,可制得浓度极高,超过1000 g / L的Ag和稳定的银水溶胶,而无需额外的稳定剂。化学,X射线光电子能谱和热重分析表明,Ag NP包含柠檬酸盐衍生的封端配体,少量的Fe适用于化学和低温烧结,表面功能化,纳米流体和其他应用。
更新日期:2020-11-23
中文翻译:

无需额外稳定剂即可制备高浓度银溶胶
银纳米颗粒(Ag NPs)在现代技术中的应用要求采用环境友好的方法,以高浓度金属溶胶和少量有机稳定剂的形式大规模生产具有受控形态和表面状态的纳米颗粒。本文中,我们报道了一种基于在柠檬酸根离子存在下用硫酸亚铁还原硝酸银水溶液的方法。我们通过应用透射电子显微镜,紫外-可见吸收光谱和动态光散射(DLS)研究了各种因素对化学反应的影响,并提出了减少试剂量的方案,从而可以制备5到15的均匀球形Ag NP直径nm。稀释后对溶胶进行DLS研究,以评估胶体颗粒相互作用的趋势,以优化合成后的纯化和浓缩程序。特别地,过滤代替离心和用柠檬酸三钠代替硝酸钠的电解凝结被用于生产极浓缩的,大于1000g / L的Ag和稳定的银水溶胶,而没有额外的稳定剂。化学,X射线光电子能谱和热重分析表明,Ag NP包含柠檬酸盐衍生的封端配体,少量的Fe适用于化学和低温烧结,表面功能化,纳米流体和其他应用。过滤代替离心分离,并用柠檬酸三钠代替硝酸钠进行电解凝结,可制得浓度极高,超过1000 g / L的Ag和稳定的银水溶胶,而无需额外的稳定剂。化学,X射线光电子能谱和热重分析表明,Ag NP包含柠檬酸盐衍生的封端配体,少量的Fe适用于化学和低温烧结,表面功能化,纳米流体和其他应用。过滤代替离心分离,并用柠檬酸三钠代替硝酸钠进行电解凝结,可制得浓度极高,超过1000 g / L的Ag和稳定的银水溶胶,而无需额外的稳定剂。化学,X射线光电子能谱和热重分析表明,Ag NP包含柠檬酸盐衍生的封端配体,少量的Fe适用于化学和低温烧结,表面功能化,纳米流体和其他应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号