Synthesis ( IF 2.6 ) Pub Date : 2020-11-12 , DOI: 10.1055/s-0040-1706568 Irina Novosjolova 1 , Māris Turks 1 , Andris Jeminejs 1 , Svetlana M. Goliškina 1 , Dmitrijs Stepanovs 2 , Ērika Bizdēna 1
|
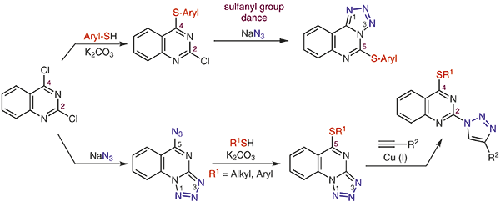
Nucleophilic aromatic substitution reaction between 4-arylthio-2-chloroquinazolines and NaN3 takes place with an unusual sulfanyl group dance and leads to the formation of 5-(arylthio)tetrazolo[1,5-c]-quinazolines, which do not form the azide tautomer and do not undergo CuAAC reactions with alkynes. On the other hand, 5-azidotetrazolo[1,5-a]quinazoline (formally described as 2,4-diazidoquinazoline) undergoes regioselective nucleophilic aromatic substitution with thiols at C5 and forms 5-(alkyl/arylthio)tetrazolo[1,5-a]quinazolines, the structure of which has been proved by X-ray crystallography. The latter exist in tautomeric equilibrium with their 2-azidoquinazoline form, which provides possibility for copper-catalyzed azide–alkyne 1,3-dipolar cycloaddition reaction, leading to the 4-alkyl/arylthio-2-(1H-1,2,3-triazol-1-yl)quinazolines.
中文翻译:

叠氮化物-四唑互变异构和芳基硫烷基团舞在硫代取代的噻唑并喹唑啉合成中的应用
4-芳硫基-2-氯喹唑啉与NaN 3之间的亲核芳族取代反应以不寻常的硫烷基跳动发生,并导致形成5-(芳硫基)四唑并[1,5- c ]-喹唑啉,而后者不形成叠氮互变异构体,不与炔烃发生CuAAC反应。另一方面,5-azidotetrazolo [1,5- a ] quinazoline (正式描述为2,4-diazidoquinazoline)在C5处用硫醇进行区域选择性亲核芳香取代,并形成5-(烷基/芳硫基)tetrazolo [1,5-一种喹唑啉,其结构已通过X射线晶体学证明。后者以2-叠氮喹啉形式互变异构平衡存在,这为铜催化的叠氮化物-炔烃1,3-偶极环加成反应提供了可能性,从而导致了4-烷基/芳硫基-2-(1 H -1,2, 3-三唑-1-基)喹唑啉。



























 京公网安备 11010802027423号
京公网安备 11010802027423号