当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrocatalytic property, anticancer activity, and density functional theory calculation of [NiCl(P^N^P)]Cl.EtOH
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2020-11-13 , DOI: 10.1002/aoc.6092 Gholamhossein Mohammadnezhad 1 , Saeed Abad 1 , Hossein Farrokhpour 1 , Helmar Görls 2 , Winfried Plass 2
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2020-11-13 , DOI: 10.1002/aoc.6092 Gholamhossein Mohammadnezhad 1 , Saeed Abad 1 , Hossein Farrokhpour 1 , Helmar Görls 2 , Winfried Plass 2
Affiliation
|
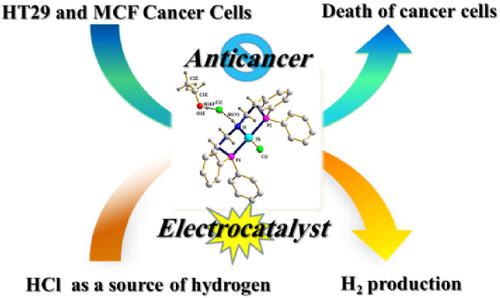
This study describes the electrocatalytic, anticancer, and density functional theory (DFT) studies of a nickel complex, [NiCl(P^N^P)]Cl.EtOH, based on a neutral P^N^P‐type pincer ligand (P^N^P = bis[(2‐diphenylphosphino)ethyl]amine). The ligand was synthesized without time‐consuming and costly amine protection. It was characterized by 1H NMR, 31P NMR, Fourier transform infrared (FT‐IR), UV–vis, and single‐crystal X‐ray diffraction. The complex was isolated as a solvated chloride salt and characterized by FT‐IR, UV–visible, 1H NMR, 13C NMR, and 31P NMR spectroscopies as well as single‐crystal X‐ray diffraction and CHN analysis. The ligand and complex crystallized in a monoclinic P21/c space group. The molecular structure of the complex contains a four‐coordinated distorted nickel ion with square‐planar geometry. The electrocatalytic hydrogen ion reduction was studied for the nickel complex in an acidic non‐aqueous medium. Cyclic voltammetry studies showed that this complex is an efficient electrocatalyst for hydrogen evolution at the potential of the Ni(II/I) couple. As a potential anticancer agent, the biological activities of the Ni complex were tested against two human cancer cell lines (MCF7 and HT29). The IC50 results demonstrated that the nickel complex has better cytotoxic activity than cis‐platin against the human breast cancer cell (MCF7) line. DFT calculations were performed to study the kinetics and thermodynamics of the pincer ligand's synthetic procedure and its Ni complex. Time‐dependent DFT calculations were performed to calculate the pincer ligand's UV–vis spectra and the complex, which was in agreement with the experimental data. To assign the calculated UV spectra, molecular orbital calculations were performed. Finally, a modified mechanism was proposed for the electrocatalytic hydrogen ion reduction by [Ni(P^N^P)Cl]Cl.EtOH. The theoretical calculations showed that the cycle is thermodynamically favorable.
中文翻译:

[NiCl(P ^ N ^ P)] Cl.EtOH的电催化性能,抗癌活性和密度泛函理论计算
这项研究描述了基于中性P ^ N ^ P型钳形配体(P的镍络合物[NiCl(P ^ N ^ P)] Cl.EtOH的电催化,抗癌和密度泛函理论(DFT)研究^ N ^ P =双[(2-二苯基膦基)乙基]胺)配体的合成没有耗时且昂贵的胺保护。它的特点是1 H NMR,31 P NMR,傅立叶变换红外(FT-IR),UV-vis和单晶X射线衍射。该复合物以溶剂化氯化物盐的形式分离,并通过FT-IR,紫外可见,1 H NMR,13 C NMR和31 P NMR光谱以及单晶X射线衍射和CHN分析进行表征。在单斜晶P 2中结晶的配体和配合物1 / c空间组。配合物的分子结构包含具有方形平面几何形状的四配位扭曲镍离子。研究了在酸性非水介质中对镍配合物的电催化氢离子还原反应。循环伏安法研究表明,该复合物在Ni(II / I)对的电势下是析氢的有效电催化剂。作为一种潜在的抗癌药,对两种人类癌细胞系(MCF7和HT29)测试了镍配合物的生物活性。IC 50结果表明,镍配合物比顺式具有更好的细胞毒活性-针对人类乳腺癌细胞(MCF7)的铂。进行DFT计算以研究钳型配体的合成过程及其Ni配合物的动力学和热力学。进行了与时间有关的DFT计算,以计算出夹钳配体的UV-vis光谱和配合物,与实验数据相符。为了分配计算的紫外线光谱,进行了分子轨道计算。最后,提出了一种通过[Ni(P ^ N ^ P)Cl] Cl.EtOH电催化还原氢离子的改进机理。理论计算表明该循环在热力学上是有利的。
更新日期:2020-11-13
中文翻译:

[NiCl(P ^ N ^ P)] Cl.EtOH的电催化性能,抗癌活性和密度泛函理论计算
这项研究描述了基于中性P ^ N ^ P型钳形配体(P的镍络合物[NiCl(P ^ N ^ P)] Cl.EtOH的电催化,抗癌和密度泛函理论(DFT)研究^ N ^ P =双[(2-二苯基膦基)乙基]胺)配体的合成没有耗时且昂贵的胺保护。它的特点是1 H NMR,31 P NMR,傅立叶变换红外(FT-IR),UV-vis和单晶X射线衍射。该复合物以溶剂化氯化物盐的形式分离,并通过FT-IR,紫外可见,1 H NMR,13 C NMR和31 P NMR光谱以及单晶X射线衍射和CHN分析进行表征。在单斜晶P 2中结晶的配体和配合物1 / c空间组。配合物的分子结构包含具有方形平面几何形状的四配位扭曲镍离子。研究了在酸性非水介质中对镍配合物的电催化氢离子还原反应。循环伏安法研究表明,该复合物在Ni(II / I)对的电势下是析氢的有效电催化剂。作为一种潜在的抗癌药,对两种人类癌细胞系(MCF7和HT29)测试了镍配合物的生物活性。IC 50结果表明,镍配合物比顺式具有更好的细胞毒活性-针对人类乳腺癌细胞(MCF7)的铂。进行DFT计算以研究钳型配体的合成过程及其Ni配合物的动力学和热力学。进行了与时间有关的DFT计算,以计算出夹钳配体的UV-vis光谱和配合物,与实验数据相符。为了分配计算的紫外线光谱,进行了分子轨道计算。最后,提出了一种通过[Ni(P ^ N ^ P)Cl] Cl.EtOH电催化还原氢离子的改进机理。理论计算表明该循环在热力学上是有利的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号