Tetrahedron ( IF 2.1 ) Pub Date : 2020-11-13 , DOI: 10.1016/j.tet.2020.131758 Jiufeng Wu , Claire M. Young , Andrew D. Smith
|
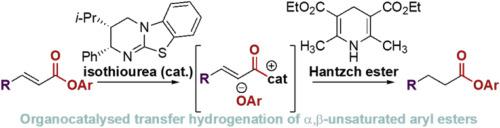
A protocol for the isothiourea-catalysed transfer hydrogenation of α,β-unsaturated para-nitrophenyl esters using Hantzsch ester has been developed. Good to excellent yields are observed using α,β-unsaturated aryl esters bearing electron-withdrawing β-substituents. The aryl ester products can either be isolated directly in moderate to excellent yields (7 examples, 16–98%) or converted to the corresponding methyl esters (2 examples, 68–70% yield) or benzyl amides (2 examples, 44–88% yield) after in situ reaction of the hydrogenated ester with the appropriate nucleophile. Preliminary experiments showed that modest enantioinduction (76:24 er) is possible when a chiral isothiourea catalyst was used.
中文翻译:

异硫脲催化的α,β-不饱和对硝基苯基酯的转移加氢
已开发出使用Hantzsch酯的异硫脲催化的α,β-不饱和对硝基苯酯转移氢化的方案。使用带有吸电子β-取代基的α,β-不饱和芳基酯,观察到良好至优异的产率。可以直接分离出芳基酯产物,以中等至优异的收率(7例,产率为16–98%)或转化为相应的甲酯(2例,产率为68–70%)或苄基酰胺(2例,44-88)。氢化酯与适当的亲核试剂进行原位反应后得到 初步实验表明,当使用手性异硫脲催化剂时,适度的对映体诱导(76:24 er)是可能的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号