Journal of Structural Biology ( IF 3 ) Pub Date : 2020-11-13 , DOI: 10.1016/j.jsb.2020.107655 Ankita Shukla 1 , Mohammad Afsar 1 , Nelam Kumar 1 , Sanjay Kumar 1 , Ravishankar Ramachandran 1
|
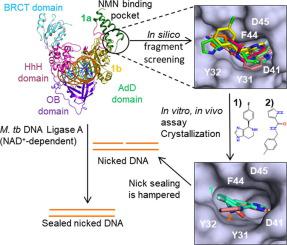
NAD+-dependent DNA ligase (LigA) is the essential replicative ligase in bacteria and differs from ATP-dependent counterparts like the human DNA ligase I (HligI) in several aspects. LigA uses NAD+ as the co-factor while the latter uses ATP. Further, the LigA carries out enzymatic activity with a single divalent metal ion in the active site while ATP-dependent ligases use two metal ions. Instead of the second metal ion, LigA have a unique NMN binding subdomain that facilitates the orientation of the β-phosphate and NMN leaving group. LigA are therefore attractive targets for new anti-bacterial therapeutic development. Others and our group have earlier identified several LigA inhibitors that mainly bind to AMP binding site of LigA. However, no inhibitor is known to bind to the unique NMN binding subdomain. We initiated a fragment inhibitor discovery campaign against the M. tuberculosis LigA based on our co-crystal structure of adenylation domain with AMP and NMN. The study identified two fragments, 4-(4-fluorophenyl)-4,5,6,7-tetrahydro-3H imidazo[4,5-c] pyridine and N-(4-methylbenzyl)-1H-pyrrole-2-carboxamide, that bind to the NMN site. The fragments inhibit LigA with IC50 of 16.9 and 28.7 µM respectively and exhibit MIC of ∼20 and 60 µg/ml against a temperature sensitive E. coli GR501 ligAts strain, rescued by MtbLigA. Co-crystal structures of the fragments with the adenylation domain of LigA show that they mimic the interactions of NMN. Overall, our results suggest that the NMN binding-site is a druggable target site for developing anti-LigA therapeutic strategies.
中文翻译:

基于结构鉴定靶向结核分枝杆菌 NAD+ 依赖性 DNA 连接酶 A 的 NMN 口袋的一流片段抑制剂
NAD +依赖性 DNA 连接酶 (LigA) 是细菌中必不可少的复制连接酶,在几个方面不同于人类 DNA 连接酶 I (HligI) 等 ATP 依赖性对应物。LigA 使用 NAD +作为辅助因子,而后者使用 ATP。此外,LigA 使用活性位点中的单个二价金属离子进行酶促活性,而 ATP 依赖性连接酶使用两种金属离子。代替第二种金属离子,LigA 具有独特的 NMN 结合子域,可促进 β-磷酸盐和 NMN 离去基团的定向。因此,LigA 是新的抗菌治疗开发的有吸引力的目标。其他人和我们小组早些时候已经确定了几种主要与 LigA 的 AMP 结合位点结合的 LigA 抑制剂。然而,已知没有抑制剂与独特的 NMN 结合子域结合。我们发起了针对结核分枝杆菌的片段抑制剂发现活动LigA 基于我们的腺苷酸化域与 AMP 和 NMN 的共晶结构。该研究鉴定了两个片段,4-(4-氟苯基)-4,5,6,7-四氢-3H咪唑并[4,5-c]吡啶和N-(4-甲基苄基)-1H-吡咯-2-甲酰胺,绑定到 NMN 站点。这些片段抑制 LigA 的 IC 50 分别为 16.9 和 28.7 µM,对温度敏感的大肠杆菌GR501 ligA ts菌株的MIC 分别为 20 和 60 µg/ml ,由 MtbLigA 拯救。片段与 LigA 腺苷酸化域的共晶结构表明它们模拟了 NMN 的相互作用。总的来说,我们的结果表明 NMN 结合位点是开发抗 LigA 治疗策略的可药物靶点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号