当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hemilabile N‐heterocyclic carbene and nitrogen ligands on Fe (II) catalyst for utilization of CO2 into cyclic carbonate
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2020-11-11 , DOI: 10.1002/aoc.6099 Fei Chen 1 , Sheng Tao 2 , Ning Liu 2 , Cheng Guo 3 , Bin Dai 1, 2
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2020-11-11 , DOI: 10.1002/aoc.6099 Fei Chen 1 , Sheng Tao 2 , Ning Liu 2 , Cheng Guo 3 , Bin Dai 1, 2
Affiliation
|
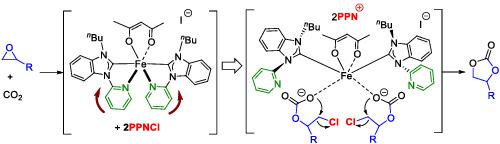
Six Fe (II) complexes were synthesized based on the concept of the hemilability of hybrid ligands, and their catalytic behaviors and performances were evaluated for the fixation of CO2 via the cycloaddition of epoxides. The catalytic potential of the Fe (II) complexes, in combination with bis(triphenylphosphoranylidene)ammonium chloride, have been proved to achieve the efficient conversion in some challenging substrates such as internal, disubstituted epoxides, oxetanes, and fatty acid‐derived epoxides for synthesis of cyclic carbonates under the mild reaction conditions. Ultraviolet–visible and in situ Fourier transform infrared spectroscopy experiments as well as our previous coordination of Ni complexes studies revealed that the origin of activity of Fe (II) complexes might be attributed to the trans effect between N‐heterocyclic carbene ligands and pyridine nitrogen donors.
中文翻译:

Fe(II)催化剂上的半不稳定N杂环卡宾和氮配体,用于将CO2利用到环状碳酸酯中
基于杂化配体半合性的概念合成了六种Fe(II)配合物,并通过环氧化物的环加成反应评估了它们的催化行为和性能,以固定CO 2。已证明,Fe(II)配合物与双(三苯基膦基亚烷基)氯化铵的催化潜力可在某些具有挑战性的底物(例如内部,双取代的环氧化物,氧杂环丁烷和脂肪酸衍生的环氧化物)中实现有效转化温和的反应条件下生成环状碳酸酯。紫外可见光和原位傅里叶变换红外光谱实验以及我们先前对镍配合物的研究表明,铁(II)配合物的活性起源可能归因于N-杂环卡宾配体与吡啶氮供体之间的反式作用。
更新日期:2020-11-11
中文翻译:

Fe(II)催化剂上的半不稳定N杂环卡宾和氮配体,用于将CO2利用到环状碳酸酯中
基于杂化配体半合性的概念合成了六种Fe(II)配合物,并通过环氧化物的环加成反应评估了它们的催化行为和性能,以固定CO 2。已证明,Fe(II)配合物与双(三苯基膦基亚烷基)氯化铵的催化潜力可在某些具有挑战性的底物(例如内部,双取代的环氧化物,氧杂环丁烷和脂肪酸衍生的环氧化物)中实现有效转化温和的反应条件下生成环状碳酸酯。紫外可见光和原位傅里叶变换红外光谱实验以及我们先前对镍配合物的研究表明,铁(II)配合物的活性起源可能归因于N-杂环卡宾配体与吡啶氮供体之间的反式作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号