当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Reduction of Oxygen by a Copper Thiosemicarbazone Complex
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2020-11-12 , DOI: 10.1002/ejic.202000869 Tatiana Straistari 1, 2, 3 , Adina Morozan 1 , Sergiu Shova 4 , Marius Réglier 2 , Maylis Orio 2 , Vincent Artero 1
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2020-11-12 , DOI: 10.1002/ejic.202000869 Tatiana Straistari 1, 2, 3 , Adina Morozan 1 , Sergiu Shova 4 , Marius Réglier 2 , Maylis Orio 2 , Vincent Artero 1
Affiliation
|
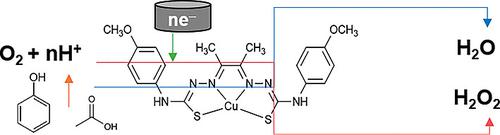
The new copper(II) thiosemicarbazone complex CuL (H2L = 4‐{bis[4‐(p‐methoxyphenyl)‐thiosemicarbazone]}‐2,3‐butane) was synthesized and structurally characterized. Its electrochemical behavior in N,N‐dimethylformamide (DMF) was examined. This complex proved active for homogeneous oxygen reduction reaction (ORR) in DMF in the presence of organic acids. The activity and selectivity for ORR were found to be dependent on the nature of the proton source (phenol or acetic acid). This study confirms the previously reported free energy linear correlation between selectivity and overpotential requirement [Passard et al., J. Am. Chem. Soc. 2016, 138, 2925–2928] and suggests that additional descriptors are required for a full understanding of the catalytic ORR behaviour.
中文翻译:

硫代氨基脲铜配合物催化还原氧
合成了新的铜(II)硫代半碳鎓络合物CuL(H 2 L = 4- {双[4-(对-甲氧基苯基)-硫代半碳酮]}-2,3-丁烷)并对其结构进行了表征。研究了其在N,N-二甲基甲酰胺(DMF)中的电化学行为。在有机酸存在下,该络合物对DMF中的均相氧还原反应(ORR)具有活性。发现ORR的活性和选择性取决于质子源(苯酚或乙酸)的性质。这项研究证实了先前报道的选择性和超电势需求之间的自由能线性相关性[Passard et al。,J. Am。化学 Soc。2016,138,2925–2928],并建议需要附加的描述符来全面了解催化ORR行为。
更新日期:2020-12-22
中文翻译:

硫代氨基脲铜配合物催化还原氧
合成了新的铜(II)硫代半碳鎓络合物CuL(H 2 L = 4- {双[4-(对-甲氧基苯基)-硫代半碳酮]}-2,3-丁烷)并对其结构进行了表征。研究了其在N,N-二甲基甲酰胺(DMF)中的电化学行为。在有机酸存在下,该络合物对DMF中的均相氧还原反应(ORR)具有活性。发现ORR的活性和选择性取决于质子源(苯酚或乙酸)的性质。这项研究证实了先前报道的选择性和超电势需求之间的自由能线性相关性[Passard et al。,J. Am。化学 Soc。2016,138,2925–2928],并建议需要附加的描述符来全面了解催化ORR行为。



























 京公网安备 11010802027423号
京公网安备 11010802027423号