European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-11-12 , DOI: 10.1016/j.ejmech.2020.113018 Eirinaios I. Vrettos , Theodoros Karampelas , Nisar Sayyad , Anastasia Kougioumtzi , Nelofer Syed , Timothy Crook , Carol Murphy , Constantin Tamvakopoulos , Andreas G. Tzakos
|
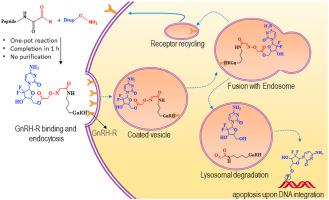
Peptide-drug conjugates (PDCs) are gaining considerable attention as anti-neoplastic agents. However, their development is often laborious and time-consuming. Herein, we have developed and preclinically evaluated three PDCs with gemcitabine as the anticancer cytotoxic unit and D-Lys6-GnRH (gonadotropin-releasing hormone; GnRH) as the cancer-targeting unit. These units were tethered via acid-labile programmable linkers to guide a differential drug release rate from the PDC through a combination of ester or amide and “click” type oxime ligations. The pro-drugs were designed to enable the selective targeting of malignant tumor cells with linker guided differential drug release rates. We exploited the oxime bond responsiveness against the acidic pH of the tumor microenvironment and the GnRH endocytosis via the GnRH-R GPCR which is overexpressed on cancer cells. The challenging metabolic properties of gemcitabine were addressed during design of the PDCs. We developed a rapid (1 hour) and cost-effective “click” oxime bond ligation platform to assemble in one-pot the 3 desired PDCs that does not require purification, surpassing traditional time-ineffective and low yield methods. The internalization of the tumor-homing peptide unit in cancer cells overexpressing the GnRH-R was first validated through confocal laser microscopy and flow cytometry analysis. Subsequently, the three PDCs were evaluated for their in vitro antiproliferative effect in prostate cancer cells. Their stability and the release of gemcitabine over time were monitored in vitro in cell culture and in human plasma using LC-MS/MS. We then assessed the ability of the developed PDCs to internalize in prostate cancer cells and to release gemcitabine. The most potent analog, designated GOXG1, was used for pharmacokinetic studies in mice. The metabolism of GOXG1 was examined in liver microsomes, as well as in buffers mimicking the pH of intracellular organelles, resulting in the identification of two metabolites. The major metabolite at low pH emanated from the cleavage of the pH-labile oxime bond, validating our design approach. NMR spectroscopy and in vitro radioligand binding assays were exploited for GOXG1 to validate that upon conjugating the drug to the peptide, the peptide microenvironment responsible for its GnRH-R binding is not perturbed and to confirm its high binding potency to the GnRH-R. Finally, the binding of GOXG1 to the GnRH-R and the associated elicitation of testosterone release in mice were also determined. The facile platform established herein for the rapid assembly of PDCs with linker controllable characteristics from aldehyde and aminooxy units through rapid “click” oxime ligation, that does not require purification steps, could pave the way for a new generation of potent cancer therapeutics, diagnostics and theranostics.
中文翻译:

带有接头可控“点击”肟键系链的可编程吉西他滨-GnRH前药的开发以及针对前列腺癌的临床前评估
肽-药物缀合物(PDC)作为抗肿瘤剂正获得相当大的关注。然而,它们的开发通常是费力且费时的。在这里,我们已经开发并临床前评估了三个吉西他滨作为抗癌细胞毒性单位和D-Lys 6的PDC。-GnRH(促性腺激素释放激素; GnRH)作为癌症靶向单位。这些单元通过对酸不敏感的可编程接头进行束缚,通过酯或酰胺与“咔嗒”型肟连接的组合来指导药物从PDC释放的差异速率。前药被设计成能够以接头引导的差异药物释放速率选择性靶向恶性肿瘤细胞。我们通过在癌细胞上过度表达的GnRH-R GPCR,利用肟键对肿瘤微环境的酸性pH和GnRH内吞作用的响应。在PDC设计过程中解决了吉西他滨具有挑战性的代谢特性。我们开发了一种快速(1小时)且经济高效的“点击”肟键连接平台,以一锅的方式组装了3个所需的不需要纯化的PDC,超越了传统的时间无效且产量低的方法。首先通过共聚焦激光显微镜和流式细胞术分析验证了过表达GnRH-R的癌细胞中肿瘤归巢肽单元的内在化。随后,对三个PDC进行了评估在前列腺癌细胞中的体外抗增殖作用。使用LC-MS / MS在细胞培养和人血浆中体外监测其稳定性和吉西他滨随时间的释放。然后,我们评估了已开发的PDC在前列腺癌细胞中内在化并释放吉西他滨的能力。最有效的类似物称为GOXG 1,用于小鼠的药代动力学研究。在肝微粒体以及模拟细胞内细胞器pH的缓冲液中检查了GOXG 1的代谢,从而鉴定了两种代谢物。低pH值肟键的裂解产生了低pH值下的主要代谢产物,这验证了我们的设计方法。NMR光谱学和体外对GOXG 1进行了放射性配体结合测定,以验证将药物与肽缀合后,负责其GnRH-R结合的肽微环境没有受到干扰,并确认了其与GnRH-R的高结合力。最后,还确定了GOXG 1与GnRH-R的结合以及小鼠中睾丸酮释放的相关诱导。通过快速的“点击”肟连接,本文建立的用于从醛和氨氧基单元快速组装具有接头可控特性的PDC的简便平台,无需纯化步骤,可以为新一代有效的癌症治疗,诊断和治疗铺平道路。治疗学。


























 京公网安备 11010802027423号
京公网安备 11010802027423号