Colloids and Surfaces B: Biointerfaces ( IF 5.8 ) Pub Date : 2020-11-12 , DOI: 10.1016/j.colsurfb.2020.111455 Hualu Lai 1 , Xin Ding 1 , Junxian Ye 1 , Jie Deng 1 , Shengmiao Cui 1
|
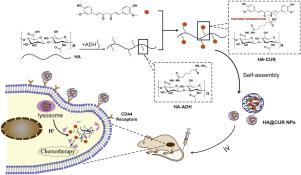
Curcumin (CUR) display promising antitumor effects, however, the poor water solubility severely limited its clinical application. To overcome this problem, polymeric nanocarriers have been adopted for targeted CUR delivery and enhanced cancer therapy. In this paper, utilizing an acid-labile hydrazone linkage, hydrophobic CUR was conjugated with hydrophilic hyaluronic acid (HA) to form amphiphilic HA-ADH-CUR conjugates, which could subsequently self-assemble to form nanoparticles (HA@CUR NPs) in aqueous. The in vitro drug release experiments showed that HA@CUR NPs exhibited a pH-responsive CUR release behavior, and the release rate of CUR was 73.5 % in pH 5.0. Further, in vitro cell experiments showed HA@CUR NPs could be efficiently internalized by 4T1 and MCF-7 cancer cells through CD44 receptor mediated endocytosis and successfully release CUR in acidic lysosome environment for chemotherapy. In vivo antitumor experiments showed that, compared to free CUR, HA@CUR NPs could efficiently cumulate in tumor site via EPR effect and CD44 mediated endocytosis, achieve superior therapeutic effect for tumor growth suppression. Therefore, HA@CUR NPs were a highly promising nanocarrier for hydrophobic CUR to realize enhanced cancer therapy with good biosafety.
中文翻译:

pH响应的透明质酸基纳米颗粒,用于姜黄素的靶向递送和增强的癌症治疗
姜黄素(CUR)具有良好的抗肿瘤作用,但是水溶性差,严重限制了其临床应用。为了克服这个问题,已经将聚合物纳米载体用于靶向的CUR递送和增强的癌症治疗。本文利用酸不稳定的an键,将疏水性CUR与亲水性透明质酸(HA)偶联形成两亲性HA-ADH-CUR偶联物,随后可在水中自组装形成纳米颗粒(HA @ CUR NPs) 。的体外药物释放实验表明,HA @ CUR的NP表现出pH-响应CUR释放行为,和CUR的释放速率在pH 5.0的为73.5%。此外,体外细胞实验表明,HA @ CUR NPs可以通过CD44受体介导的内吞作用被4T1和MCF-7癌细胞有效内化,并在酸性溶酶体环境中成功释放CUR进行化疗。体内抗肿瘤实验表明,与游离CUR相比,HA @ CUR NPs可以通过EPR效应和CD44介导的内吞作用在肿瘤部位有效积聚,达到抑制肿瘤生长的优异治疗效果。因此,HA @ CUR NPs是疏水CUR的高度有前途的纳米载体,可以实现具有良好生物安全性的增强癌症治疗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号