Cancer Letters ( IF 9.7 ) Pub Date : 2020-11-11 , DOI: 10.1016/j.canlet.2020.11.002 Noélia Che 1 , Kai-Yu Ng 1 , Tin-Lok Wong 1 , Man Tong 2 , Phillis Wf Kau 1 , Lok-Hei Chan 1 , Terence K Lee 3 , Michael Sy Huen 1 , Jing-Ping Yun 4 , Stephanie Ma 2
|
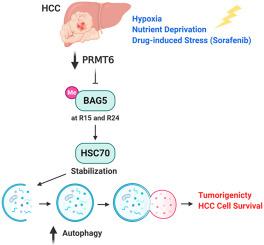
Autophagy is a critical survival factor for cancer cells, whereby it maintains cellular homeostasis by degrading damaged organelles and unwanted proteins and supports cellular biosynthesis in response to stress. Cancer cells, including hepatocellular carcinoma (HCC), are often situated in a hypoxic, nutrient-deprived and stressful microenvironment where tumor cells are yet still able to adapt and survive. However, the mechanism underlying this adaptation and survival is not well-defined. We report deficiency of the post-translational modification enzyme protein arginine N-methyltransferase 6 (PRMT6) in HCC to promote the induction of autophagy under oxygen/nutrient-derived and sorafenib drug-induced stress conditions. Enhanced autophagic flux in HCC cells negatively correlated with PRMT6 expression, with the catalytic domain of PRMT6 critically important in mediating these autophagic activities. Mechanistically, PRMT6 physically interacts and methylates BAG5 to enhance the degradation of its interacting partner HSC70, a well-known autophagy player. The therapeutic potential of targeting BAG5 using genetic approach to reverse tumorigenicity and sorafenib resistance mediated by PRMT6 deficiency in HCC is also demonstrated in an in vivo model. The clinical implications of these findings are highlighted by the inverse correlative expressions of PRMT6 and HSC70 in HCC tissues. Collectively, deficiency of PRMT6 induces autophagy to promote tumorigenicity and cell survival in hostile microenvironments of HCC tumors by regulating BAG5-associated HSC70 stability through post-translational methylation of BAG5. Targeting BAG5 may therefore be an attractive strategy in HCC treatment by suppressing autophagy and inducing HCC cell sensitivity to sorafenib for treatment.
中文翻译:

PRMT6缺陷通过调节BAG5相关HSC70稳定性诱导肝细胞癌肿瘤微环境中的自噬
自噬是癌细胞的关键存活因子,它通过降解受损细胞器和不需要的蛋白质来维持细胞稳态,并支持细胞生物合成以应对压力。癌细胞,包括肝细胞癌 (HCC),通常位于缺氧、营养缺乏和压力大的微环境中,肿瘤细胞仍然能够适应和生存。然而,这种适应和生存的机制尚不明确。我们报告了 HCC 中翻译后修饰酶蛋白精氨酸 N-甲基转移酶 6 (PRMT6) 的缺乏,以促进在氧气/营养来源和索拉非尼药物诱导的应激条件下诱导自噬。HCC 细胞中增强的自噬通量与 PRMT6 表达负相关,PRMT6 的催化域在介导这些自噬活动中至关重要。从机制上讲,PRMT6 与 BAG5 发生物理相互作用并使 BAG5 甲基化,以增强其相互作用伙伴 HSC70(一种著名的自噬参与者)的降解。使用遗传方法靶向 BAG5 以逆转 HCC 中由 PRMT6 缺陷介导的致瘤性和索拉非尼耐药性的治疗潜力也在一项研究中得到证实。体内模型。这些发现的临床意义通过 PRMT6 和 HSC70 在 HCC 组织中的负相关表达得到强调。总的来说,PRMT6 的缺乏通过 BAG5 的翻译后甲基化调节 BAG5 相关的 HSC70 稳定性,诱导自噬促进 HCC 肿瘤的敌对微环境中的致瘤性和细胞存活。因此,通过抑制自噬和诱导 HCC 细胞对索拉非尼治疗的敏感性,靶向 BAG5 可能是 HCC 治疗中一种有吸引力的策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号