当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Desymmetrization of meso-Dicarbonatecyclohexene with β-Hydrazino Carboxylic Esters via a Pd-Catalyzed Allylic Substitution Cascade
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.orglett.0c03211 Kai Xu 1 , Yan Zheng 1 , Yong Ye 2 , Delong Liu 1 , Wanbin Zhang 1, 2
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.orglett.0c03211 Kai Xu 1 , Yan Zheng 1 , Yong Ye 2 , Delong Liu 1 , Wanbin Zhang 1, 2
Affiliation
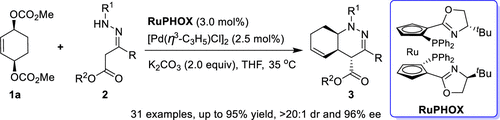
|
The desymmetrization of meso-dicarbonatecyclohexene with β-hydrazino carboxylic esters has been achieved via a RuPHOX/Pd-catalyzed allylic substitution cascade for the construction of chiral hexahydrocinnoline derivatives with high performance. Mechanistic studies reveal that the reaction exploits a pathway different from that of our previous work and that the first nitrogen nucleophilic process is the rate-determining step. The protocol could be conducted on a gram scale without any loss of catalytic behavior, and the corresponding chiral hexahydrocinnolines can undergo diverse transformations.
中文翻译:

的Desymmetrization内消旋-通过Pd催化的烯丙基取代级联与β肼羧酸酯Dicarbonatecyclohexene
的desymmetrization内消旋-dicarbonatecyclohexene与β -肼基羧酸酯已通过已经实现一个RuPHOX / Pd催化的烯丙基取代级联为与高性能的手性hexahydrocinnoline衍生物的构建。机理研究表明,该反应采用了不同于我们先前工作的途径,并且第一个氮亲核过程是决定速率的步骤。该方案可以以克为单位进行,而没有任何催化行为的损失,并且相应的手性六氢肉桂碱可以经历各种转化。
更新日期:2020-11-21
中文翻译:

的Desymmetrization内消旋-通过Pd催化的烯丙基取代级联与β肼羧酸酯Dicarbonatecyclohexene
的desymmetrization内消旋-dicarbonatecyclohexene与β -肼基羧酸酯已通过已经实现一个RuPHOX / Pd催化的烯丙基取代级联为与高性能的手性hexahydrocinnoline衍生物的构建。机理研究表明,该反应采用了不同于我们先前工作的途径,并且第一个氮亲核过程是决定速率的步骤。该方案可以以克为单位进行,而没有任何催化行为的损失,并且相应的手性六氢肉桂碱可以经历各种转化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号