Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective Synthesis of Monodisperse CoO Nanooctahedra as Catalysts for Electrochemical Water Oxidation
Langmuir ( IF 3.9 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.langmuir.0c02131 Massih Sarif 1 , Jan Hilgert 1 , Ibrahim Khan 2 , Richard A. Harris 3 , Sergi Plana-Ruiz 4, 5 , Muhammad Ashraf 6 , Eva Pütz 1 , Jörg Schemberg 7 , Martin Panthöfer 1 , Ute Kolb 1 , Muhammad Nawaz Tahir 6 , Wolfgang Tremel 1
Langmuir ( IF 3.9 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.langmuir.0c02131 Massih Sarif 1 , Jan Hilgert 1 , Ibrahim Khan 2 , Richard A. Harris 3 , Sergi Plana-Ruiz 4, 5 , Muhammad Ashraf 6 , Eva Pütz 1 , Jörg Schemberg 7 , Martin Panthöfer 1 , Ute Kolb 1 , Muhammad Nawaz Tahir 6 , Wolfgang Tremel 1
Affiliation
|
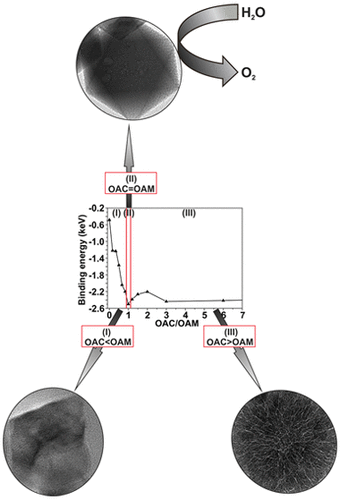
Thermal decomposition is a promising route for the synthesis of metal oxide nanoparticles because size and morphology can be tuned by minute control of the reaction variables. We synthesized CoO nanooctahedra with diameters of ∼48 nm and a narrow size distribution. Full control over nanoparticle size and morphology could be obtained by controlling the reaction time, surfactant ratio, and reactant concentrations. We show that the particle size does not increase monotonically with time or surfactant concentration but passes through minima or maxima. We unravel the critical role of the surfactants in nucleation and growth and rationalize the observed experimental trends in accordance with simulation experiments. The as-synthesized CoO nanooctahedra exhibit superior electrocatalytic activity with long-term stability during oxygen evolution. The morphology of the CoO particles controls the electrocatalytic reaction through the distinct surface sites involved in the oxygen evolution reaction.
中文翻译:

选择性合成单分散CoO纳米八面体作为电化学水氧化催化剂
热分解是合成金属氧化物纳米颗粒的有前途的途径,因为可以通过对反应变量的精细控制来调节其大小和形态。我们合成了直径约48 nm且尺寸分布窄的CoO纳米八面体。通过控制反应时间,表面活性剂比例和反应物浓度,可以完全控制纳米颗粒的大小和形态。我们表明,粒径不会随时间或表面活性剂浓度而单调增加,而是通过最小值或最大值。我们阐明了表面活性剂在成核和生长中的关键作用,并根据模拟实验合理化了观察到的实验趋势。合成后的CoO纳米八面体在氧气析出过程中具有出色的电催化活性和长期稳定性。
更新日期:2020-11-25
中文翻译:

选择性合成单分散CoO纳米八面体作为电化学水氧化催化剂
热分解是合成金属氧化物纳米颗粒的有前途的途径,因为可以通过对反应变量的精细控制来调节其大小和形态。我们合成了直径约48 nm且尺寸分布窄的CoO纳米八面体。通过控制反应时间,表面活性剂比例和反应物浓度,可以完全控制纳米颗粒的大小和形态。我们表明,粒径不会随时间或表面活性剂浓度而单调增加,而是通过最小值或最大值。我们阐明了表面活性剂在成核和生长中的关键作用,并根据模拟实验合理化了观察到的实验趋势。合成后的CoO纳米八面体在氧气析出过程中具有出色的电催化活性和长期稳定性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号